Genetic susceptibility and caspase activation in mouse and human macrophages are distinct for Legionella longbeachae and L. pneumophila
- PMID: 17261610
- PMCID: PMC1865702
- DOI: 10.1128/IAI.00025-07
Genetic susceptibility and caspase activation in mouse and human macrophages are distinct for Legionella longbeachae and L. pneumophila
Abstract
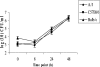
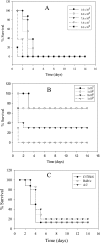
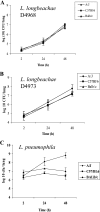
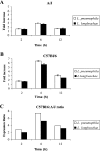
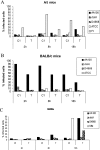
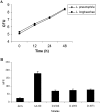
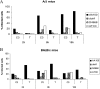
Legionella pneumophila is the predominant cause of Legionnaires' disease in the United States and Europe, while Legionella longbeachae is the common cause of the disease in Western Australia. Although clinical manifestations by both intracellular pathogens are very similar, recent studies have shown that phagosome biogeneses of both species within human macrophages are distinct (R. Asare and Y. Abu Kwaik, Cell. Microbiol., in press). Most inbred mouse strains are resistant to infection by L. pneumophila, with the exception of the A/J mouse strain, and this genetic susceptibility is associated with polymorphism in the naip5 allele and flagellin-mediated early activation of caspase 1 and pyropoptosis in nonpermissive mouse macrophages. Here, we show that genetic susceptibility of mice to infection by L. longbeachae is independent of allelic polymorphism of naip5. L. longbeachae replicates within bone marrow-derived macrophages and in the lungs of A/J, C57BL/6, and BALB/c mice, while L. pneumophila replicates in macrophages in vitro and in the lungs of the A/J mouse strain only. Quantitative real-time PCR studies on infected A/J and C57BL/6 mouse bone marrow-derived macrophages show that both L. longbeachae and L. pneumophila trigger similar levels of naip5 expression, but the levels are higher in infected C57BL/6 mouse macrophages. In contrast to L. pneumophila, L. longbeachae has no detectable pore-forming activity and does not activate caspase 1 in A/J and C57BL/6 mouse or human macrophages, despite flagellation. Unlike L. pneumophila, L. longbeachae triggers only a modest activation of caspase 3 and low levels of apoptosis in human and murine macrophages in vitro and in the lungs of infected mice at late stages of infection. We conclude that despite flagellation, infection by L. longbeachae is independent of polymorphism in the naip5 allele and L. longbeachae does not trigger the activation of caspase 1, caspase 3, or late-stage apoptosis in mouse and human macrophages. Neither species triggers caspase 1 activation in human macrophages.
Figures




Similar articles
-
Global cellular changes induced by Legionella pneumophila infection of bone marrow-derived macrophages.Immunobiology. 2011 Dec;216(12):1274-85. doi: 10.1016/j.imbio.2011.06.008. Epub 2011 Jun 30. Immunobiology. 2011. PMID: 21794945
-
Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection.PLoS Pathog. 2009 Apr;5(4):e1000361. doi: 10.1371/journal.ppat.1000361. Epub 2009 Apr 3. PLoS Pathog. 2009. PMID: 19343209 Free PMC article.
-
Caspase-1 but Not Caspase-11 Is Required for NLRC4-Mediated Pyroptosis and Restriction of Infection by Flagellated Legionella Species in Mouse Macrophages and In Vivo.J Immunol. 2015 Sep 1;195(5):2303-11. doi: 10.4049/jimmunol.1501223. Epub 2015 Jul 31. J Immunol. 2015. PMID: 26232428
-
Naip5/Birc1e and susceptibility to Legionella pneumophila.Trends Microbiol. 2005 Jul;13(7):328-35. doi: 10.1016/j.tim.2005.05.007. Trends Microbiol. 2005. PMID: 15935674 Review.
-
[Control of intracellular Legionella pneumophila growth--with special reference to the Lgn1/Naip5/Birc1e gene--].Nihon Saikingaku Zasshi. 2009 Dec;64(2-4):319-30. doi: 10.3412/jsb.64.319. Nihon Saikingaku Zasshi. 2009. PMID: 19628930 Review. Japanese. No abstract available.
Cited by
-
Concept about the Virulence Factor of Legionella.Microorganisms. 2022 Dec 27;11(1):74. doi: 10.3390/microorganisms11010074. Microorganisms. 2022. PMID: 36677366 Free PMC article. Review.
-
Ectopic Expression of Human Thymosin β4 Confers Resistance to Legionella pneumophila during Pulmonary and Systemic Infection in Mice.Infect Immun. 2021 Mar 17;89(4):e00735-20. doi: 10.1128/IAI.00735-20. Print 2021 Mar 17. Infect Immun. 2021. PMID: 33468581 Free PMC article.
-
Virulence factors encoded by Legionella longbeachae identified on the basis of the genome sequence analysis of clinical isolate D-4968.J Bacteriol. 2010 Feb;192(4):1030-44. doi: 10.1128/JB.01272-09. Epub 2009 Dec 11. J Bacteriol. 2010. PMID: 20008069 Free PMC article.
-
Legionella pneumophila strain 130b possesses a unique combination of type IV secretion systems and novel Dot/Icm secretion system effector proteins.J Bacteriol. 2010 Nov;192(22):6001-16. doi: 10.1128/JB.00778-10. Epub 2010 Sep 10. J Bacteriol. 2010. PMID: 20833813 Free PMC article.
-
The unique Legionella longbeachae capsule favors intracellular replication and immune evasion.PLoS Pathog. 2024 Sep 11;20(9):e1012534. doi: 10.1371/journal.ppat.1012534. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39259722 Free PMC article.
References
-
- Abu-Zant, A., S. Jones, R. Asare, J. Suttles, C. Price, J. Graham, and Y. A. Kwaik. 2007. Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell. Microbiol. 9:246-264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

