Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae
- PMID: 17261615
- PMCID: PMC1865667
- DOI: 10.1128/IAI.01435-06
Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae
Abstract
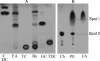
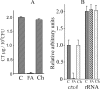
Bile induces pleiotropic responses that affect production of virulence factors, motility, and other phenotypes in the enteric pathogen Vibrio cholerae. Since bile is a heterogeneous mixture, crude bile was fractionated, and the components that mediate virulence gene repression and enhancement of motility were identified by nuclear magnetic resonance, gas chromatography (GC), and GC-mass spectrometry analyses. The unsaturated fatty acids detected in bile, arachidonic, linoleic, and oleic acids, drastically repressed expression of the ctxAB and tcpA genes, which encode cholera toxin and the major subunit of the toxin-coregulated pilus, respectively. The unsaturated fatty acid-dependent repression was due to silencing of ctxAB and tcpA expression by the histone-like nucleoid-structuring protein H-NS, even in the presence of the transcriptional activator ToxT. Unsaturated fatty acids also enhanced motility of V. cholerae due to increased expression of flrA, the first gene of a regulatory cascade that controls motility. H-NS had no role in the fatty acid-mediated enhancement of motility. It is likely that the ToxR/ToxT system that negatively regulates motility is rendered nonfunctional in the presence of unsaturated fatty acids, leading to an increase in motility. Motility and flrA expression were also increased in the presence of cholesterol, another component of bile, in an H-NS- and ToxR/ToxT-independent manner.
Figures





References
-
- Ausbel, F. M., R. R. Brent, E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. John Wiley and Sons, New York, NY.
-
- Gunn, J. S. 2000. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2:907-913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

