Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae
- PMID: 17261616
- PMCID: PMC1865688
- DOI: 10.1128/IAI.01758-06
Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae
Abstract
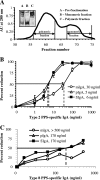
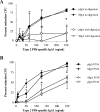
Antibodies of the immunoglobulin A (IgA) class react with capsular polysaccharides of Streptococcus pneumoniae and support complement-dependent opsonophagocytosis (OPC) of the organism by phagocytes. We characterized the biologic impact of the molecular forms of human monoclonal capsule-specific IgA (monomeric IgA [mIgA], polymeric IgA [pIgA], and secretory IgA [SIgA]) on OPC and susceptibility to cleavage by IgA1 protease. The efficiency of SIgA in support of OPC of S. pneumoniae was comparable to that of pIgA, and both forms exceeded that of mIgA by a fivefold margin. This structure-function relationship was associated with three factors. First, the avidities, or functional affinities, of both pIgA and SIgA for pneumococcal capsules exceeded those of mIgA. Second, both pIgA and SIgA required less complement to achieve similar levels of bacterial OPC than did mIgA, indicating that secretory component does not hinder the effect of complement. Third, both pIgA and SIgA mediated agglutination of the organism, whereas mIgA did not. All three forms of capsule-specific IgA showed comparable susceptibilities to cleavage and functional inhibition by bacterial IgA1 protease, demonstrating that secretory component does not prevent the proteolytic degradation of IgA1 by IgA1 protease. IgA1 cleavage results in formation of identical Fab fragments for each of the molecular forms, thereby abolishing the contribution of multivalence of pIgA and SIgA. In summary, the polymeric forms of IgA (both pIgA and SIgA) provide a substantial advantage in binding, agglutination, and OPC of the organism.
Figures





References
-
- Bachmann, M. F., A. Kalinke, A. Althage, G. Freer, C. Burkhart, H.-P. Roost, H. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 276:2024-2027. - PubMed
-
- Berdoz, J., and B. Corthésy. 2004. Human polymeric IgA is superior to IgG and single-chain Fv of the same monoclonal specificity to inhibit urease activity associated with Helicobacter pylori. Mol. Immunol. 41:1013-1022. - PubMed
-
- Bogers, W. M. J. M., R.-K. Stad, L. A. van Es, and M. R. Daha. 1991. Immunoglobulin A: interaction with complement, phagocytic cells and endothelial cells. Complement Inflamm. 8:347-358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE 15844/DE/NIDCR NIH HHS/United States
- P30 DK034928/DK/NIDDK NIH HHS/United States
- R21 AI044231/AI/NIAID NIH HHS/United States
- R01 HD041361/HD/NICHD NIH HHS/United States
- AI 48796/AI/NIAID NIH HHS/United States
- P30 DK 34928/DK/NIDDK NIH HHS/United States
- R37 AI038446/AI/NIAID NIH HHS/United States
- R21 DE015072/DE/NIDCR NIH HHS/United States
- R01 AI048796/AI/NIAID NIH HHS/United States
- R01 AI038446/AI/NIAID NIH HHS/United States
- HD 41361/HD/NICHD NIH HHS/United States
- R01 AI044231/AI/NIAID NIH HHS/United States
- AI 44231/AI/NIAID NIH HHS/United States
- AI 38446/AI/NIAID NIH HHS/United States
- DE 015072/DE/NIDCR NIH HHS/United States
- R01 DE015844/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

