Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation
- PMID: 17261633
- PMCID: PMC2118720
- DOI: 10.1084/jem.20062319
Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation
Abstract
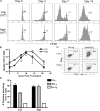
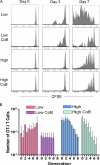
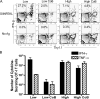
After a brief period of antigenic stimulation, T cells become committed to a program of autonomous expansion and differentiation. We investigated the role of antigen-specific T cell precursor frequency as a possible cell-extrinsic factor impacting T cell programming in a model of allogeneic tissue transplantation. Using an adoptive transfer system to incrementally raise the precursor frequency of antigen-specific CD8(+) T cells, we found that donor-reactive T cells primed at low frequency exhibited increased cellular division, decreased development of multifunctional effector activity, and an increased requirement for CD28- and CD154-mediated costimulation relative to those primed at high frequency. The results demonstrated that recipients with low CD4(+) and CD8(+) donor-reactive T cell frequencies exhibited long-term skin graft survival upon CD28/CD154 blockade, whereas simultaneously raising the frequency of CD4(+) T cells to approximately 0.5% and CD8(+) T cells to approximately 5% precipitated graft rejection despite CD28/CD154 blockade. Antigenic rechallenge of equal numbers of cells stimulated at high or low frequency revealed that cells retained an imprint of the frequency at which they were primed. These results demonstrate a critical role for initial precursor frequency in determining the CD8(+) T cell requirement for CD28- and CD154-mediated costimulatory signals during graft rejection.
Figures



Similar articles
-
A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection.J Immunol. 2008 Jun 1;180(11):7203-11. doi: 10.4049/jimmunol.180.11.7203. J Immunol. 2008. PMID: 18490719 Free PMC article.
-
Different costimulatory and growth factor requirements for CD4+ and CD8+ T cell-mediated rejection.J Immunol. 2004 Jul 1;173(1):214-21. doi: 10.4049/jimmunol.173.1.214. J Immunol. 2004. PMID: 15210777
-
High-frequency alloreactive T cells augment effector function of low-frequency CD8+ T-cell responses under CD28/CD154 blockade.Transplantation. 2010 May 27;89(10):1208-17. doi: 10.1097/TP.0b013e3181df53dc. Transplantation. 2010. PMID: 20407401 Free PMC article.
-
Critical role of OX40 in CD28 and CD154-independent rejection.J Immunol. 2004 Feb 1;172(3):1691-8. doi: 10.4049/jimmunol.172.3.1691. J Immunol. 2004. PMID: 14734751
-
Advances in transplantation tolerance.Lancet. 2001 Jun 16;357(9272):1959-63. doi: 10.1016/s0140-6736(00)05068-6. Lancet. 2001. PMID: 11425437 Review.
Cited by
-
T Cell Cosignaling Molecules in Transplantation.Immunity. 2016 May 17;44(5):1020-33. doi: 10.1016/j.immuni.2016.04.012. Immunity. 2016. PMID: 27192567 Free PMC article. Review.
-
Targeting Metabolism as a Platform for Inducing Allograft Tolerance in the Absence of Long-Term Immunosuppression.Front Immunol. 2020 Apr 9;11:572. doi: 10.3389/fimmu.2020.00572. eCollection 2020. Front Immunol. 2020. PMID: 32328063 Free PMC article.
-
2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses.J Exp Med. 2014 Feb 10;211(2):297-311. doi: 10.1084/jem.20130902. Epub 2014 Feb 3. J Exp Med. 2014. PMID: 24493803 Free PMC article.
-
CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice.J Immunol. 2008 Sep 15;181(6):3906-14. doi: 10.4049/jimmunol.181.6.3906. J Immunol. 2008. PMID: 18768845 Free PMC article.
-
Differential requirements of CD4(+) T-cell signals for effector cytotoxic T-lymphocyte (CTL) priming and functional memory CTL development at higher CD8(+) T-cell precursor frequency.Immunology. 2013 Apr;138(4):298-306. doi: 10.1111/imm.12033. Immunology. 2013. PMID: 23113741 Free PMC article.
References
-
- Arakelov, A., and F.G. Lakkis. 2000. The alloimmune response and effector mechanisms of allograft rejection. Semin. Nephrol. 20:95–102. - PubMed
-
- Khoury, S., M.H. Sayegh, and L.A. Turka. 1999. Blocking costimulatory signals to induce transplantation tolerance and prevent autoimmune disease. Int. Rev. Immunol. 18:185–199. - PubMed
-
- Salomon, B., and J.A. Bluestone. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19:225–252. - PubMed
-
- Quezada, S.A., L.Z. Jarvinen, E.F. Lind, and R.J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

