The role of estrogens in normal and abnormal development of the prostate gland
- PMID: 17261752
- PMCID: PMC2276871
- DOI: 10.1196/annals.1386.009
The role of estrogens in normal and abnormal development of the prostate gland
Abstract
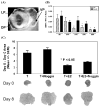
Estrogens play a physiologic role during prostate development with regard to programming stromal cells and directing early morphogenic events. However, if estrogenic exposures are abnormally high during the critical developmental period, permanent alterations in prostate branching morphogenesis and cellular differentiation will result, a process referred to as neonatal imprinting or developmental estrogenization. These perturbations are associated with an increased incidence of prostatic lesions with aging, which include hyperplasia, inflammation, and dysplasia. To understand how early estrogenic exposures can permanently alter the prostate and predispose it to neoplasia, we examined the effects of estrogens on prostatic steroid receptors and key developmental genes. Transient and permanent alterations in prostatic AR, ERalpha, ERbeta, and RARs are observed. We propose that estrogen-induced alterations in these critical transcription factors play a fundamental role in initiating prostatic growth and differentiation defects by shifting the prostate from an androgen-dominated gland to one whose development is regulated by estrogens and retinoids. This in turn leads to specific disruptions in the expression patterns of key prostatic developmental genes that normally dictate morphogenesis and differentiation. Specifically, we find transient reductions in Nkx3.1 and permanent reductions in Hoxb-13, which lead to differentiation defects particularly within the ventral lobe. Prolonged developmental expression of Bmp-4 contributes to hypomorphic growth throughout the prostatic complex. Reduced expression of Fgf10 and Shh and their cognate receptors in the dorsolateral lobes leads to branching defects in those specific regions in response to neonatal estrogens. We hypothesize that these molecular changes initiated early in life predispose the prostate to the neoplastic state upon aging.
Figures

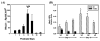
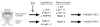
Similar articles
-
Influence of neonatal estrogens on rat prostate development.Reprod Fertil Dev. 2001;13(4):241-52. doi: 10.1071/rd00107. Reprod Fertil Dev. 2001. PMID: 11800163
-
The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogens.Dev Biol. 2005 Feb 15;278(2):396-414. doi: 10.1016/j.ydbio.2004.11.020. Dev Biol. 2005. PMID: 15680359
-
Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure.Endocrinology. 2002 Sep;143(9):3628-40. doi: 10.1210/en.2002-220184. Endocrinology. 2002. PMID: 12193579
-
The role of estrogens and estrogen receptors in normal prostate growth and disease.Steroids. 2008 Mar;73(3):233-44. doi: 10.1016/j.steroids.2007.10.013. Epub 2007 Nov 12. Steroids. 2008. PMID: 18093629 Free PMC article. Review.
-
Developmental estrogen exposures predispose to prostate carcinogenesis with aging.Reprod Toxicol. 2007 Apr-May;23(3):374-82. doi: 10.1016/j.reprotox.2006.10.001. Epub 2006 Oct 24. Reprod Toxicol. 2007. PMID: 17123779 Free PMC article. Review.
Cited by
-
Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT.Mol Endocrinol. 2010 May;24(5):993-1006. doi: 10.1210/me.2009-0438. Epub 2010 Mar 29. Mol Endocrinol. 2010. PMID: 20351197 Free PMC article.
-
Novel biomarkers for risk of prostate cancer: results from a case-control study.Prostate. 2009 Jan 1;69(1):41-8. doi: 10.1002/pros.20850. Prostate. 2009. PMID: 18816637 Free PMC article.
-
Cyclooxygenase-2 activates EGFR-ERK1/2 pathway via PGE2-mediated ADAM-17 signaling in testosterone-induced benign prostatic hyperplasia.Inflammopharmacology. 2023 Feb;31(1):499-516. doi: 10.1007/s10787-022-01123-7. Epub 2022 Dec 31. Inflammopharmacology. 2023. PMID: 36586043 Free PMC article.
-
Heparan sulfate inhibits transforming growth factor β signaling and functions in cis and in trans to regulate prostate stem/progenitor cell activities.Glycobiology. 2020 May 19;30(6):381-395. doi: 10.1093/glycob/cwz103. Glycobiology. 2020. PMID: 31829419 Free PMC article.
-
Veterinary growth promoters in cattle feedlot runoff: estrogenic activity and potential effects on the rat male reproductive system.Environ Sci Pollut Res Int. 2020 Apr;27(12):13939-13948. doi: 10.1007/s11356-020-07966-3. Epub 2020 Feb 8. Environ Sci Pollut Res Int. 2020. PMID: 32034597
References
-
- Lasnitzki I, Mizuno T. Antagonistic effects of cyproterone acetate and oestradiol on the development of the fetal rat prostate gland induced by androgens in organ culture. Prostate. 1980;1:147–156. - PubMed
-
- Price D. Normal development of the prostate and seminal vesicles of the rat with a study of experimental postnatal modifications. Am J Anat. 1936;60:79–127.
-
- Zondek LH, Zondek T. Congenital malformations of the male accessory sex glands in the fetus and neonate. In: Spring-Mills E, Hafez ESE, editors. Male Accessory Sex Glands. Elsevier–North Holland; New York: 1980. pp. 17–37.
-
- Adams JY, Leav I, Lau KM, et al. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52:69–81. - PubMed
-
- Jarred RA, McPherson SJ, Bianco JJ, et al. Prostate phenotypes in estrogen-modulated transgenic mice. Trends Endocrinol Metab. 2002;13:163–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

