Antioxidant defense response in a galling insect
- PMID: 17261812
- PMCID: PMC1783901
- DOI: 10.1073/pnas.0604722104
Antioxidant defense response in a galling insect
Abstract
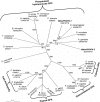
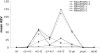
Herbivorous insect species are constantly challenged with reactive oxygen species (ROS) generated from endogenous and exogenous sources. ROS produced within insects because of stress and prooxidant allelochemicals produced by host plants in response to herbivory require a complex mode of antioxidant defense during insect/plant interactions. Some insect herbivores have a midgut-based defense against the suite of ROS encountered. Because the Hessian fly (Mayetiola destructor) is the major insect pest of wheat worldwide, and an emerging model for all gall midges, we investigated its antioxidant responses during interaction with its host plant. Quantitative data for two phospholipid glutathione peroxidases (MdesPHGPX-1 and MdesPHGPX-2), two catalases (MdesCAT-1 and MdesCAT-2), and two superoxide dismutases (MdesSOD-1 and MdesSOD-2) revealed high levels of all of the mRNAs in the midgut of larvae on susceptible wheat (compatible interaction). During development of the Hessian fly on susceptible wheat, a differential expression pattern was observed for all six genes. Analysis of larvae on resistant wheat (incompatible interaction) compared with larvae on susceptible wheat showed increased levels of mRNAs in larvae on resistant wheat for all of the antioxidant genes except MdesSOD-1 and MdesSOD-2. We postulate that the increased mRNA levels of MdesPHGPX-1, MdesPHGPX-2, MdesCAT-1, and MdesCAT-2 reflect responses to ROS encountered by larvae while feeding on resistant wheat seedlings and/or ROS generated endogenously in larvae because of stress/starvation. These results provide an opportunity to understand the cooperative antioxidant defense responses in the Hessian fly/wheat interaction and may be applicable to other insect/plant interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
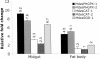
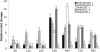
Similar articles
-
Hessian fly larval feeding triggers enhanced polyamine levels in susceptible but not resistant wheat.BMC Plant Biol. 2015 Jan 16;15:3. doi: 10.1186/s12870-014-0396-y. BMC Plant Biol. 2015. PMID: 25592131 Free PMC article.
-
Reactive oxygen species are involved in plant defense against a gall midge.Plant Physiol. 2010 Feb;152(2):985-99. doi: 10.1104/pp.109.150656. Epub 2009 Dec 4. Plant Physiol. 2010. PMID: 19965963 Free PMC article.
-
Phenotypic and molecular characterization of Hessian fly resistance in diploid wheat, Aegilops tauschii.BMC Plant Biol. 2019 Oct 22;19(1):439. doi: 10.1186/s12870-019-2058-6. BMC Plant Biol. 2019. PMID: 31640550 Free PMC article.
-
Gall midges (Hessian flies) as plant pathogens.Annu Rev Phytopathol. 2012;50:339-57. doi: 10.1146/annurev-phyto-072910-095255. Epub 2012 May 29. Annu Rev Phytopathol. 2012. PMID: 22656645 Review.
-
Grasses and gall midges: plant defense and insect adaptation.Annu Rev Entomol. 2003;48:549-77. doi: 10.1146/annurev.ento.48.091801.112559. Epub 2002 Jun 4. Annu Rev Entomol. 2003. PMID: 12460937 Review.
Cited by
-
Pseudomonas syringae enhances herbivory by suppressing the reactive oxygen burst in Arabidopsis.J Insect Physiol. 2016 Jan;84:90-102. doi: 10.1016/j.jinsphys.2015.07.011. Epub 2015 Jul 21. J Insect Physiol. 2016. PMID: 26205072 Free PMC article.
-
Unraveling the tapestry of networks involving reactive oxygen species in plants.Plant Physiol. 2008 Jul;147(3):978-84. doi: 10.1104/pp.108.122325. Plant Physiol. 2008. PMID: 18612075 Free PMC article. No abstract available.
-
The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line.PLoS One. 2008 May 7;3(5):e2083. doi: 10.1371/journal.pone.0002083. PLoS One. 2008. PMID: 18461124 Free PMC article.
-
ApCarE4 and ApPOD3 participate in the adaptation of pea aphids to different alfalfa varieties.Sci Rep. 2024 Oct 26;14(1):25444. doi: 10.1038/s41598-024-76192-5. Sci Rep. 2024. PMID: 39455643 Free PMC article.
-
Spermidine supplementation influence on protective enzymes of Apis mellifera (Hymenoptera: Apidae).J Insect Sci. 2024 Sep 1;24(5):3. doi: 10.1093/jisesa/ieae098. J Insect Sci. 2024. PMID: 39382172 Free PMC article.
References
-
- Ahmad S, Pardini RS. Free Radical Biol Med. 1990;8:401–413. - PubMed
-
- Barbehenn RV. J Chem Ecol. 2002;28:1329–1347. - PubMed
-
- Maiorino M, Scapin M, Ursini F, Biasolo M, Bosello V, Flohe L. J Biol Chem. 2003;278:34286–34290. - PubMed
-
- Behne D, Kyriakopoulos A. Annu Rev Nutr. 2001;21:453–473. - PubMed
-
- Tang L, Gounaris K, Griffiths C, Selkirk E. J Biol Chem. 1995;270:18313–18318. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

