Cytoprotective effects of proteasome beta5 subunit overexpression in lens epithelial cells
- PMID: 17262013
- PMCID: PMC2503187
Cytoprotective effects of proteasome beta5 subunit overexpression in lens epithelial cells
Abstract
Purpose: To determine whether the overexpression of the proteasome catalytic beta5 subunit (PSMB5) can induce the expression of the catalytic subunits beta1 and beta2, enhance proteasome activity, and exert a cytoprotective effect in lens epithelial cells.
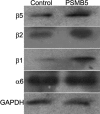
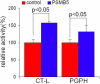
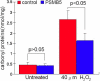
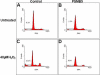
Methods: Cells from the human lens epithelial cell line SRA01/04 (LECs) were stably transfected either with a plasmid expressing the proteasome catalytic subunit beta5 or with an empty plasmid. beta-5-expressing LECs and controls were analyzed for the expression of beta1, beta2, beta5, and alpha6 proteasome subunits; chymotrypsin-like (CT-L) and peptidylglutamyl-peptide hydrolase (PGPH) catalytic activities; as well as for the accumulation of carbonylated proteins, rates of cell viability, and apoptosis after oxidative stress.
Results: Stable expression of the beta5 proteasome subunit resulted in increased expression of the catalytic subunits beta1 and beta2, increased CT-L and PGPH proteasome activities, and increased resistance to accumulation of carbonylated proteins and cell death after oxidative stress.
Conclusions: The proteasome activity can be genetically "upregulated" in lens cells by overexpression of the beta5 catalytic subunit. The resulting increase in proteasome activity leads to a decrease in the accumulation of oxidized proteins and enhanced cell survival following oxidative stress.
Figures

Similar articles
-
Apigenin, chrysin, and luteolin selectively inhibit chymotrypsin-like and trypsin-like proteasome catalytic activities in tumor cells.Planta Med. 2010 Feb;76(2):128-32. doi: 10.1055/s-0029-1186004. Epub 2009 Aug 3. Planta Med. 2010. PMID: 19653143
-
The proteasome and intracellular redox status: implications for apoptotic regulation in lens epithelial cells.Curr Eye Res. 2007 Oct;32(10):871-82. doi: 10.1080/02713680701642327. Curr Eye Res. 2007. PMID: 17963107
-
Proteasome beta-type subunits: unequal roles of propeptides in core particle maturation and a hierarchy of active site function.J Mol Biol. 1999 Aug 27;291(4):997-1013. doi: 10.1006/jmbi.1999.2995. J Mol Biol. 1999. PMID: 10452902
-
A single amino acid substitution in a proteasome subunit triggers aggregation of ubiquitinated proteins in stressed neuronal cells.J Neurochem. 2004 Jul;90(1):19-28. doi: 10.1111/j.1471-4159.2004.02456.x. J Neurochem. 2004. PMID: 15198663
-
Characterization of the 20S proteasome in human glioblastomas.Anticancer Res. 2005 Sep-Oct;25(5):3203-10. Anticancer Res. 2005. PMID: 16101128
Cited by
-
Protein quality control and degradation in cardiomyocytes.J Mol Cell Cardiol. 2008 Jul;45(1):11-27. doi: 10.1016/j.yjmcc.2008.03.025. Epub 2008 May 20. J Mol Cell Cardiol. 2008. PMID: 18495153 Free PMC article. Review.
-
The role of PSMB5 in sodium arsenite-induced oxidative stress in L-02 cells.Cell Stress Chaperones. 2020 May;25(3):533-540. doi: 10.1007/s12192-020-01104-1. Epub 2020 Apr 16. Cell Stress Chaperones. 2020. PMID: 32301004 Free PMC article.
-
Genetic and pharmacologic proteasome augmentation ameliorates Alzheimer's-like pathology in mouse and fly APP overexpression models.Sci Adv. 2022 Jun 10;8(23):eabk2252. doi: 10.1126/sciadv.abk2252. Epub 2022 Jun 8. Sci Adv. 2022. PMID: 35675410 Free PMC article.
-
PSMB5 Alleviates Ulcerative Colitis by Inhibiting ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis.Dis Markers. 2022 Aug 26;2022:2329904. doi: 10.1155/2022/2329904. eCollection 2022. Dis Markers. 2022. Retraction in: Dis Markers. 2023 Jul 19;2023:9827206. doi: 10.1155/2023/9827206. PMID: 36061354 Free PMC article. Retracted.
-
Enhancement of ubiquitin conjugation activity reduces intracellular aggregation of V76D mutant γD-crystallin.Invest Ophthalmol Vis Sci. 2012 Sep 25;53(10):6655-65. doi: 10.1167/iovs.12-9744. Invest Ophthalmol Vis Sci. 2012. PMID: 22915036 Free PMC article.
References
-
- Kuck JF, Kuck KD. The Emory mouse cataract: loss of soluble protein, glutathione, protein sulfhydryl and other changes. Exp Eye Res. 1983;36:351–62. - PubMed
-
- Garland D. Role of site-specific, metal-catalyzed oxidation in lens aging and cataract: a hypothesis. Exp Eye Res. 1990;50:677–82. - PubMed
-
- Bron AJ, Vrensen GF, Koretz J, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214:86–104. - PubMed
-
- Spector A. The search for a solution to senile cataracts. Proctor lecture. Invest Ophthalmol Vis Sci. 1984;25:130–46. - PubMed
-
- Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
