Genetic analysis of two Indian families affected with congenital hereditary endothelial dystrophy: two novel mutations in SLC4A11
- PMID: 17262014
- PMCID: PMC2503190
Genetic analysis of two Indian families affected with congenital hereditary endothelial dystrophy: two novel mutations in SLC4A11
Abstract
Purpose: The autosomal recessive form of congenital hereditary endothelial dystrophy (CHED2) is a rare eye disorder caused by mutations in the SLC4A11 gene located at the CHED2 locus on chromosome 20p13-p12. The purpose of this study was to carry out genetic analysis of CHED2 in two Indian families.
Methods: Blood samples were collected from individuals for genomic DNA isolation. In order to see if these families had mutations in the SLC4A11 gene, we selected 11 microsatellite markers from the CHED2 candidate region and used them to genotype the families. DNA sequence analysis was used for mutation detection. Allele-specific PCR was used to determine the segregation of mutations in families and also to determine if the mutations were present in 100 ethnically matched normal control chromosomes.
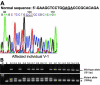
Results: Haplotype analysis suggested linkage of the disorder to the CHED2 locus in both families. DNA sequence analysis showed a novel indel mutation, c.859_862delGAGAinsCCT (E287fsX21) in exon 8 of the SLC4A11 gene in one family. This mutation is predicted to truncate the protein with a lack of all 14 transmembrane domains. DNA sequence analysis of the second family showed a novel in-frame deletion mutation c.2014_2016delTTC or 2017_2019delTTC which will lead to the loss of a phenylalanine residue at position 672 or 673 (F672del or F673del). The mutant protein is expected to lack a conserved phenylalanine residue in transmembrane domain number 8.
Conclusions: This study reports two novel mutations in two CHED2 families and increases the spectrum of mutations in SLC4A11 to a total of 16. PCR-based screening methods were developed for both mutations for rapid screening of individuals.
Figures



Similar articles
-
Missense mutation in SLC4A11 in two Pakistani families affected with congenital hereditary endothelial dystrophy (CHED2).Clin Exp Optom. 2016 Jan;99(1):73-7. doi: 10.1111/cxo.12276. Epub 2015 Aug 18. Clin Exp Optom. 2016. PMID: 26286922
-
Mutation analysis of the SLC4A11 gene in Indian families with congenital hereditary endothelial dystrophy 2 and a review of the literature.Mol Vis. 2013 Aug 2;19:1694-706. Print 2013. Mol Vis. 2013. PMID: 23922488 Free PMC article. Review.
-
Identification of mutations in the SLC4A11 gene in patients with recessive congenital hereditary endothelial dystrophy.Arch Ophthalmol. 2008 May;126(5):700-8. doi: 10.1001/archopht.126.5.700. Arch Ophthalmol. 2008. PMID: 18474783
-
Congenital hereditary endothelial dystrophy - mutation analysis of SLC4A11 and genotype-phenotype correlation in a North Indian patient cohort.Mol Vis. 2010 Dec 31;16:2955-63. Mol Vis. 2010. PMID: 21203343 Free PMC article.
-
Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome).Orphanet J Rare Dis. 2008 Oct 15;3:28. doi: 10.1186/1750-1172-3-28. Orphanet J Rare Dis. 2008. PMID: 18922146 Free PMC article. Review.
Cited by
-
Biosynthetic and functional defects in newly identified SLC4A11 mutants and absence of COL8A2 mutations in Fuchs endothelial corneal dystrophy.J Hum Genet. 2014 Aug;59(8):444-53. doi: 10.1038/jhg.2014.55. Epub 2014 Jul 10. J Hum Genet. 2014. PMID: 25007886
-
The IC3D classification of the corneal dystrophies.Cornea. 2008 Dec;27 Suppl 2(Suppl 2):S1-83. doi: 10.1097/ICO.0b013e31817780fb. Cornea. 2008. PMID: 19337156 Free PMC article. Review.
-
Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities.J Biol Chem. 2009 Sep 25;284(39):26882-96. doi: 10.1074/jbc.M109.008102. Epub 2009 Jul 8. J Biol Chem. 2009. PMID: 19586905 Free PMC article.
-
Conditionally Immortal Slc4a11-/- Mouse Corneal Endothelial Cell Line Recapitulates Disrupted Glutaminolysis Seen in Slc4a11-/- Mouse Model.Invest Ophthalmol Vis Sci. 2017 Jul 1;58(9):3723-3731. doi: 10.1167/iovs.17-21781. Invest Ophthalmol Vis Sci. 2017. PMID: 28738416 Free PMC article.
-
Exome Sequencing Reveals SLC4A11 Variant Underlying Congenital Hereditary Endothelial Dystrophy (CHED2) Misdiagnosed as Congenital Glaucoma.Genes (Basel). 2023 Jan 25;14(2):310. doi: 10.3390/genes14020310. Genes (Basel). 2023. PMID: 36833236 Free PMC article.
References
-
- Hand CK, Harmon DL, Kennedy SM, FitzSimon JS, Collum LM, Parfrey NA. Localization of the gene for autosomal recessive congenital hereditary endothelial dystrophy (CHED2) to chromosome 20 by homozygosity mapping. Genomics. 1999;61:1–4. - PubMed
-
- Toma NM, Ebenezer ND, Inglehearn CF, Plant C, Ficker LA, Bhattacharya SS. Linkage of congenital hereditary endothelial dystrophy to chromosome 20. Hum Mol Genet. 1995;4:2395–8. - PubMed
-
- Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet. 2006;38:755–7. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
