A phase I/II study of irinotecan when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group Study
- PMID: 17262086
- PMCID: PMC2360056
- DOI: 10.1038/sj.bjc.6603570
A phase I/II study of irinotecan when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group Study
Abstract
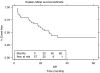
The objective of this study was to evaluate the maximum tolerated dose (MTD) and recommended dose of irinotecan administered as a 5-day schedule synchronously with 5-fluorouracil (5FU), leucovorin (LV) and preoperative pelvic radiation (45 Gy) for primary borderline/unresectable, locally advanced rectal cancer. The study used escalating doses of intravenous irinotecan (6, 8, 10, 12, 14, 16, 18, and 20 mg m(-2)) administered on days 1-5 and 29-33 followed by low dose LV (20 mg m(-2)) and 5FU (350 mg m(-2) over 1 h) in sequential cohorts. Preoperative pelvic radiotherapy using a three- or four-field technique and megavoltage photons comprised 45 Gy given in 25 fractions, 1.8 Gy per fraction. Surgery in the form of mesorectal excision was performed 6-10 weeks later. Histopathological examination of the resected specimen was performed according to techniques of Quirke, and compared with clinical staging. A distance of 1 mm or less between the peripheral extent of the tumour and the radial resection margin defined an involved circumferential resection margin (CRM). The MTD was determined as the dose causing more than a third of patients to have a dose-limiting toxicity (DLT) defined as specific grade 3 or 4 toxicities. Once the MTD was reached, a further 14 patients were treated at the dose level below the MTD. In total, 57 patients received irinotecan at the eight dose levels. The final cohort reached DLT after only four patients had been enrolled. The median age was 62 years (range 26-75), 37 male and 20 female subjects. The MTD of irinotecan in this schedule was 20 mg m(-2) when three out of four patients experienced DLT. Dose limiting grade 3 or 4 diarrhoea was reported in seven out of 57 patients, three at the 20 mg m(-2) dose level. Serious haematological toxicity (grade 3) was minimal and reported in only three patients; one grade 3 neutropaenia, one grade 4 neutropaenia and one grade 3 febrile neutropaenia and anaemia. Compliance was good with 93 and 89% of patients completing radiotherapy and chemotherapy, respectively. The remaining patients had only minor deviations from protocol therapy. Eight patients did not proceed to surgery, in six cases because they remained unresectable or had developed metastatic disease, one patient was unfit for surgery and one died as a result of complications from radiotherapy. Forty-nine patients underwent a potentially curative surgical resection. Histopathological examination of the resected specimen demonstrated pCR 12 out of 49 (24%) and 12 out of 57 (21%) overall. A histologically confirmed clear circumferential resection margin (CRM) was achieved in 39 out of 49 (80%) of those resected, and 39 out of 57 (68%) overall. In conclusion, MTD with this scheduled regimen of irinotecan is 20 mg m(-2) (days 1-5 and 29-33). The acceptable toxicity and compliance at 18 mg m(-2) recommend testing this dose in future phase III studies. The tumour downstaging and complete resection rates (negative CRM) are encouragingly high for this very locally advanced group.
Figures
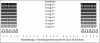
 Leucovorin 20 mg m−2 bolus days 1–5, 29–33;
Leucovorin 20 mg m−2 bolus days 1–5, 29–33;  5FU 350 mg m−2 60 min infusion days 1–5, 29–33; ↓ radiotherapy 1.8 Gy per fraction.
5FU 350 mg m−2 60 min infusion days 1–5, 29–33; ↓ radiotherapy 1.8 Gy per fraction.
Similar articles
-
A phase I/II study of oxaliplatin when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group (CCOG) study.Br J Cancer. 2005 Oct 31;93(9):993-8. doi: 10.1038/sj.bjc.6602818. Br J Cancer. 2005. PMID: 16249791 Free PMC article. Clinical Trial.
-
Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2015 Aug;16(8):979-89. doi: 10.1016/S1470-2045(15)00159-X. Epub 2015 Jul 15. Lancet Oncol. 2015. PMID: 26189067 Clinical Trial.
-
Oxaliplatin plus irinotecan and leucovorin-modulated 5-fluorouracil triplet regimen every other week: a dose-finding study in patients with advanced gastrointestinal malignancies.Ann Oncol. 2002 Dec;13(12):1874-81. doi: 10.1093/annonc/mdf307. Ann Oncol. 2002. PMID: 12453855 Clinical Trial.
-
Phase I study of weekly CPT-11 (irinotecan)/docetaxel in patients with advanced solid tumors.Lung Cancer. 2002 Aug;37(2):213-8. doi: 10.1016/s0169-5002(02)00081-8. Lung Cancer. 2002. PMID: 12140145 Review.
-
Improving the toxicity of irinotecan/5-FU/leucovorin: a 21-day schedule.Oncology (Williston Park). 2003 Sep;17(9 Suppl 8):37-43. Oncology (Williston Park). 2003. PMID: 14569847 Review.
Cited by
-
Systematic review of treatment intensification using novel agents for chemoradiotherapy in rectal cancer.Br J Surg. 2018 Nov;105(12):1553-1572. doi: 10.1002/bjs.10993. Br J Surg. 2018. PMID: 30311641 Free PMC article.
-
Early Systemic Failure After Preoperative Chemoradiotherapy for the Treatment of Patients With Rectal Cancer.Ann Coloproctol. 2019 Apr;35(2):94-99. doi: 10.3393/ac.2018.08.28. Epub 2019 Apr 30. Ann Coloproctol. 2019. PMID: 31113174 Free PMC article.
-
Irinotecan+5-fluorouracil with concomitant pre-operative radiotherapy in locally advanced non-resectable rectal cancer: a phase I/II study.Br J Cancer. 2008 Apr 8;98(7):1210-6. doi: 10.1038/sj.bjc.6604292. Epub 2008 Mar 18. Br J Cancer. 2008. PMID: 18349840 Free PMC article. Clinical Trial.
-
Clinical and cellular roles for TDP1 and TOP1 in modulating colorectal cancer response to irinotecan.Mol Cancer Ther. 2015 Feb;14(2):575-85. doi: 10.1158/1535-7163.MCT-14-0762. Epub 2014 Dec 18. Mol Cancer Ther. 2015. PMID: 25522766 Free PMC article.
-
Adjuvant therapy for rectal cancer.Clin Colon Rectal Surg. 2007 Aug;20(3):167-81. doi: 10.1055/s-2007-984861. Clin Colon Rectal Surg. 2007. PMID: 20011198 Free PMC article.
References
-
- Becerra CR, Cho LC, Gregorcyk S, Simmang C, Frenkel EP, Verma UN (2005) A phase I study with irinotecan, capecitabine and radiation therapy for patients with locally advanced or metastatic rectal cancer. Proc Am Soc Clin Oncol GI Symposium (abstract 212)
-
- Birbeck KF, Quirke P (1999) Reporting protocols in colorectal cancer. CPD Bull Cell Pathol 1: 58–64
-
- Boscia RE, Korbut T, Holden SA, Ara G, Teicher BA (1993) Interaction of topoisomerase I inhibitors with radiation in cis-diamminedichloroplatinum (II)-sensitive and -resistant cells in vitro and in the FSAIIC fibrosarcoma in vivo. Int J Cancer 53: 118–123 - PubMed
-
- Bosset JF, Calais G, Daban A, Berger C, Radosevic-Jelic L, Maingon P, Bardet E, Pierart M, Briffaux A (2004) Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance. Report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur J Cancer 40: 219–224 - PubMed
-
- Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Briffaux A, Collette L (2005a) Enhanced tumoricidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results – EORTC 22921. J Clin Oncol 23: 5620–5627 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

