The hepatitis C virus NS3 protein: a model RNA helicase and potential drug target
- PMID: 17263143
- PMCID: PMC3571657
The hepatitis C virus NS3 protein: a model RNA helicase and potential drug target
Abstract
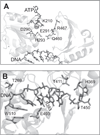
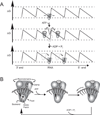
The C-terminal portion of hepatitis C virus (HCV) nonstructural protein 3 (NS3) forms a three domain polypeptide that possesses the ability to travel along RNA or single-stranded DNA (ssDNA) in a 3' to 5' direction. Fueled byATP hydrolysis, this movement allows the protein to displace complementary strands of DNA or RNA and proteins bound to the nucleic acid. HCV helicase shares two domains common to other motor proteins, one of which appears to rotate upon ATP binding. Several models have been proposed to explain how this conformational change leads to protein movement and RNA unwinding, but no model presently explains all existing experimental data. Compounds recently reported to inhibit HCV helicase, which include numerous small molecules, RNA aptamers and antibodies, will be useful for elucidating the role of a helicase in positive-sense single-stranded RNA virus replication and might serve as templates for the design of novel antiviral drugs.
Figures


References
-
- Ahmadian MR, Stege P, Scheffzek K, Wittinghofer A. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nat. Struct. Biol. 1997;4:686–689. - PubMed
-
- Artsaenko O, Tessmann K, Sack M, Haussinger D, Heintges T. Abrogation of hepatitis C virus NS3 helicase enzymatic activity by recombinant human antibodies. J. Gen. Virol. 2003;84:2323–2332. - PubMed
-
- Astumian RD. Thermodynamics and kinetics of a Brownian motor. Science. 1997;276:917–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

