Polymeric sulfated amino acid surfactants: a class of versatile chiral selectors for micellar electrokinetic chromatography (MEKC) and MEKC-MS
- PMID: 17263313
- PMCID: PMC2569972
- DOI: 10.1021/ac061228t
Polymeric sulfated amino acid surfactants: a class of versatile chiral selectors for micellar electrokinetic chromatography (MEKC) and MEKC-MS
Abstract
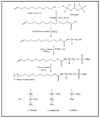
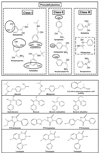
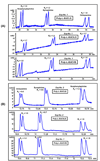
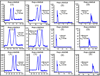
In this work, three amino acid-derived (l-leucinol, l-isoleucinol, l-valinol) sulfated chiral surfactants are synthesized and polymerized. These chiral sulfated surfactants are thoroughly characterized to determine critical micelle concentration, aggregation number, polarity, optical rotation, and partial specific volume. For the first time the morphological behavior of polymeric sulfated surfactants is revealed using cryogenic high-resolution electron microscopy. The polysodium N-undecenoyl-l-leucine sulfate shows distinct tubular structure, while polysodium N-undecenoyl-l-valine sulfate also shows tubular morphology but without any distinct order of the tubes. On the other hand, polysodium N-undecenoyl-l-isoleucine sulfate (poly-l-SUCILS) displays random distribution of coiled/curved filaments with heavy association of tightly and loosely bound water. All three polymeric sulfated surfactants are compared for enantioseparation of a broad range of structurally diverse racemic compounds at very acidic, neutral, and basic pH conditions in micellar electrokinetic chromatography (MEKC). A small combinatorial library of 10 structurally related phenylethylamines (PEAs) is investigated for chiral separation under acidic and moderately acidic to neutral pH conditions using an experimental design. In contrast to neutral pH conditions, at acidic pH, significantly enhanced chiral resolution is obtained for class I and class II PEAs due to the compact structure of polymeric sulfated surfactants. It is observed that the presence of a hydroxy group on the benzene ring of PEAs resulted in deterioration of enantioseparation. A sensitive MEKC-mass spectrometry (MS) method is developed for one of the PEAs (e.g., (+/-)-pseudoephedrine) in human urine. Very low limit of detection (LOD) is obtained at pH 2.0 (LOD 325 ng/mL), which is approximately 16 times better compared to pH 8.0 (LOD 5.2 microg/mL). Another broad range of chiral analytes (beta-blockers, phenoxypropionic acid, benzoin derivatives, PTH-amino acids, benzodiazepinones) studied also provided improved chiral separation at low pH compared to high-pH conditions. Among the three polymeric sulfated surfactants, poly-l-SUCILS with two chiral centers on the polymer head group provided overall higher enantioresolution for the investigated acidic, basic, and neutral compounds. This work clearly demonstrates for the first time the superiority of chiral separation and sensitive MS detection at low pH over conventional high-pH chiral separation and detection employing anionic chiral polymeric surfactants in MEKC and MEKC-MS.
Figures









Similar articles
-
Polymeric alkenoxy amino acid surfactants: V. Comparison of carboxylate and sulfate head group polymeric surfactants for enantioseparation in MEKC.Electrophoresis. 2007 Jun;28(11):1762-78. doi: 10.1002/elps.200600483. Electrophoresis. 2007. PMID: 17480038
-
Carbohydrate-Based Polymeric Surfactants for Chiral Micellar Electrokinetic Chromatography (CMEKC) Coupled to Mass Spectrometry.Methods Mol Biol. 2019;1985:417-444. doi: 10.1007/978-1-4939-9438-0_25. Methods Mol Biol. 2019. PMID: 31069750 Free PMC article.
-
Synthesis, characterization, and application of chiral ionic liquids and their polymers in micellar electrokinetic chromatography.Anal Chem. 2006 Oct 1;78(19):7061-9. doi: 10.1021/ac060878u. Anal Chem. 2006. PMID: 17007537 Free PMC article.
-
Enantiomer separation of drugs by micellar electrokinetic chromatography using chiral surfactants.J Chromatogr A. 2000 Apr 14;875(1-2):163-78. doi: 10.1016/s0021-9673(99)01167-x. J Chromatogr A. 2000. PMID: 10839143 Review.
-
Chiral electrokinetic chromatography using dipeptide polymeric surfactants: present state of the art.Electrophoresis. 1999 Oct;20(15-16):3011-26. doi: 10.1002/(SICI)1522-2683(19991001)20:15/16<3011::AID-ELPS3011>3.0.CO;2-#. Electrophoresis. 1999. PMID: 10596813 Review.
Cited by
-
Capillary electrophoresis in bioanalysis.Anal Chem. 2008 Jun 15;80(12):4533-50. doi: 10.1021/ac8007384. Epub 2008 May 17. Anal Chem. 2008. PMID: 18484738 Free PMC article. Review. No abstract available.
-
Determination of enantiomeric compositions of analytes using novel fluorescent chiral molecular micelles and steady state fluorescence measurements.J Fluoresc. 2008 Mar;18(2):285-96. doi: 10.1007/s10895-007-0268-z. Epub 2007 Nov 6. J Fluoresc. 2008. PMID: 17985217 Free PMC article.
-
Separation and determination of warfarin enantiomers in human plasma using a novel polymeric surfactant for micellar electrokinetic chromatography-mass spectrometry.J Chromatogr A. 2007 Aug 3;1159(1-2):208-16. doi: 10.1016/j.chroma.2007.04.037. Epub 2007 Apr 21. J Chromatogr A. 2007. PMID: 17499757 Free PMC article.
-
Multivariate approach for the enantioselective analysis in micellar electrokinetic chromatography-mass spectrometry. I. Simultaneous optimization of binaphthyl derivatives in negative ion mode.J Chromatogr A. 2009 Jan 30;1216(5):845-56. doi: 10.1016/j.chroma.2008.11.093. Epub 2008 Dec 6. J Chromatogr A. 2009. PMID: 19110258 Free PMC article.
-
Establishing repeatability and ruggedness of chiral separations in micellar electrokinetic chromatography mass spectrometry: Combined use of covalently bonded capillary column and molecular micelles.J Chromatogr A. 2020 Apr 26;1617:460835. doi: 10.1016/j.chroma.2019.460835. Epub 2019 Dec 30. J Chromatogr A. 2020. PMID: 31928773 Free PMC article.
References
-
- Davankov VA. Pure Appl. Chem. 1997;69:1469–1474.
-
- Ward TJ, Hamburg DM. Anal. Chem. 2004;76:4635–4644. - PubMed
-
- Beesley TE, Scott RP. Chiral Chromatography. New York: John Wiley & Sons; 1999.
-
- Aboul-Enein HY, editor. Chiral Separations by Liquid Chromatography: Theory and Applications (Chromatographic Science, Vol. 90) (Chromatographic Science) Boca Raton, FL: CRC-Press; 2003.
-
- Gubitz G, Schmid MG, editors. Chiral Separations: Methods and Protocols (Methods in Molecular Biology) New Jersey: Humana Press; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

