Trends in the development and approval of monoclonal antibodies for viral infections
- PMID: 17263584
- PMCID: PMC7100544
- DOI: 10.2165/00063030-200721010-00001
Trends in the development and approval of monoclonal antibodies for viral infections
Abstract
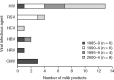
Monoclonal antibodies (mAbs) developed for either the prevention or treatment of viral diseases represent a small, but valuable, class of products. Since 1985, commercial firms have initiated clinical studies involving a total of 28 mAbs. To date, one product (palivizumab) has been approved and eight candidates are currently in clinical study. Most commercial mAbs studied as antiviral agents in the clinic have either directly or indirectly targeted human immunodeficiency virus, respiratory syncytial virus, or hepatitis C virus infections. However, the ability of mAbs to bind to specific targets and utilize various anti-infective modes of action would seem to make them well suited for the prevention and/or treatment of a wider variety of viral diseases. A number of factors, including the continuing need for innovative medicines for viral infections, the global spread of viral infections, and increased government funding for the study of pathogen countermeasures, have prompted companies to reconsider mAbs as antiviral agents. Public sector research into the use of mAbs against emerging pathogens, such as severe acute respiratory syndrome coronavirus, may have already provided candidates for further development.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

