Effect of chloroquine on reducing HIV-1 replication in vitro and the DC-SIGN mediated transfer of virus to CD4+ T-lymphocytes
- PMID: 17263871
- PMCID: PMC1796897
- DOI: 10.1186/1742-4690-4-6
Effect of chloroquine on reducing HIV-1 replication in vitro and the DC-SIGN mediated transfer of virus to CD4+ T-lymphocytes
Abstract
Background: Chloroquine (CQ) has been shown to inhibit HIV-1 replication in vitro as well as in vivo and has been proposed to alter the glycosylation pattern of the gp120 envelope. These activities indicate that the compound can be used not only as an effective HIV-1 therapeutic agent but also as a modulator of the gp120 envelope protein structure enabling for the production of broader neutralizing Ab responses.
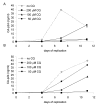
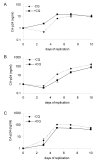
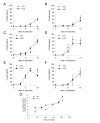
Results: We confirm here that HIV-1 replication on CD4+ T-lymphocytes can be reduced in the presence of CQ and show that the reduced replication is producer cell mediated, with viruses generated in the presence of CQ not being inhibited for subsequent infectivity and replication. By analysing the gp120 envelope protein sequences from viruses cultured long-term in the absence or presence of CQ we demonstrate variant evolution patterns. One noticeable change is the reduction in the number of potential N-linked glycosylation sites in the V3 region as well as within the 2G12 Ab binding and neutralization epitope. We also demonstrate that HIV-1 produced in the presence of CQ has a reduced capacity for transfer by Raji-DC-SIGN cells to CD4+ T-lymphocytes, indicating another means whereby virus transmission or replication may be reduced in vivo.
Conclusion: These results indicate that CQ should be considered as an HIV-1 therapeutic agent with its influence exerted through a number of mechanisms in vivo, including modulation of the gp120 structure.
Figures
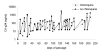

Similar articles
-
Interaction of HIV-1 with dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin-expressing cells is influenced by gp120 envelope modifications associated with disease progression.FEBS J. 2006 Nov;273(21):4944-58. doi: 10.1111/j.1742-4658.2006.05491.x. Epub 2006 Sep 28. FEBS J. 2006. PMID: 17010165
-
Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes.J Clin Invest. 2005 Nov;115(11):3256-64. doi: 10.1172/JCI25105. Epub 2005 Oct 20. J Clin Invest. 2005. PMID: 16239964 Free PMC article.
-
Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells.Antimicrob Agents Chemother. 2006 Oct;50(10):3367-74. doi: 10.1128/AAC.00593-06. Antimicrob Agents Chemother. 2006. PMID: 17005819 Free PMC article.
-
The anti-HIV-1 activity of chloroquine.J Clin Virol. 2001 Feb;20(3):131-5. doi: 10.1016/s1386-6532(00)00139-6. J Clin Virol. 2001. PMID: 11166661 Review.
-
DC-T cell virological synapses and the skin: novel perspectives in dermatology.Exp Dermatol. 2015 Jan;24(1):1-4. doi: 10.1111/exd.12511. Epub 2014 Nov 11. Exp Dermatol. 2015. PMID: 25039899 Review.
Cited by
-
Immune Modulation as a Therapeutic Option During the SARS-CoV-2 Outbreak: The Case for Antimalarial Aminoquinolines.Front Immunol. 2020 Aug 28;11:2159. doi: 10.3389/fimmu.2020.02159. eCollection 2020. Front Immunol. 2020. PMID: 32983179 Free PMC article. Review.
-
Efficacy of chloroquine versus lopinavir/ritonavir in mild/general COVID-19 infection: a prospective, open-label, multicenter, randomized controlled clinical study.Trials. 2020 Jul 8;21(1):622. doi: 10.1186/s13063-020-04478-w. Trials. 2020. PMID: 32641091 Free PMC article. Clinical Trial.
-
A multi-centre, randomized, double-blind, placebo-controlled clinical trial of the efficacy and safety of chloroquine phosphate, hydroxychloroquine sulphate and lopinavir/ritonavir for the treatment of COVID-19 in Lagos State: study protocol for a randomized controlled trial.Trials. 2021 Dec 4;22(1):869. doi: 10.1186/s13063-021-05675-x. Trials. 2021. PMID: 34863267 Free PMC article.
-
Protein glycosylation in infectious disease pathobiology and treatment.Cent Eur J Biol. 2011;6(5):802. doi: 10.2478/s11535-011-0050-8. Epub 2011 Aug 10. Cent Eur J Biol. 2011. PMID: 32215117 Free PMC article. Review.
-
BCA2/Rabring7 targets HIV-1 Gag for lysosomal degradation in a tetherin-independent manner.PLoS Pathog. 2014 May 22;10(5):e1004151. doi: 10.1371/journal.ppat.1004151. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24852021 Free PMC article.
References
-
- Savarino A, Lucia MB, Rastrelli E, Rutella S, Golotta C, Morra E, Tamburrini E, Perno CF, Boelaert JR, Sperber K, Cauda R. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J Acquir Immune Defic Syndr. 2004;35:223–232. - PubMed
-
- Koch M, Pancera M, Kwong PD, Kolchinsky P, Grundner C, Wang L, Hendrickson WA, Sodroski J, Wyatt R. Structure-based, targeted deglycosylation of HIV-1 gp120 and effects on neutralization sensitivity and antibody recognition. Virol. 2003;313:387–400. doi: 10.1016/S0042-6822(03)00294-0. - DOI - PubMed
-
- Lekkerkerker AN, Ludwig IS, van Vliet SJ, van Kooyk Y, Geijtenbeek TB. Potency of HIV-1 envelope glycoprotein gp120 antibodies to inhibit the interaction of DC-SIGN with HIV-1 gp120. Virol. 2004;329:465–476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

