E2F-1 induces melanoma cell apoptosis via PUMA up-regulation and Bax translocation
- PMID: 17263886
- PMCID: PMC1797184
- DOI: 10.1186/1471-2407-7-24
E2F-1 induces melanoma cell apoptosis via PUMA up-regulation and Bax translocation
Abstract
Background: PUMA is a pro-apoptotic Bcl-2 family member that has been shown to be involved in apoptosis in many cell types. We sought to ascertain whether induction of PUMA plays a crucial role in E2F-1-induced apoptosis in melanoma cells.
Methods: PUMA gene and protein expression levels were detected by real-time PCR and Western blot in SK-MEL-2 and HCT116 cell lines after Ad-E2F-1 infection. Activation of the PUMA promoter by E2F-1 overexpression was detected by dual luciferase reporter assay. E2F-1-induced Bax translocation was shown by immunocytochemistry. The induction of caspase-9 activity was measured by caspase-9 colorimetric assay kit.
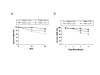
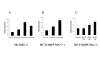
Results: Up-regulation of the PUMA gene and protein by E2F-1 overexpression was detected by real-time PCR and Western blot analysis in the SK-MEL-2 melanoma cell line. In support of this finding, we found six putative E2F-1 binding sites within the PUMA promoter. Subsequent dual luciferase reporter assay showed that E2F-1 expression could increase the PUMA gene promoter activity 9.3 fold in SK-MEL-2 cells. The role of PUMA in E2F-1-induced apoptosis was further investigated in a PUMA knockout cell line. Cell viability assay showed that the HCT116 PUMA-/- cell line was more resistant to Ad-E2F-1-mediated cell death than the HCT116 PUMA+/+ cell line. Moreover, a 2.2-fold induction of the PUMA promoter was also noted in the HCT116 PUMA+/+ colon cancer cell line after Ad-E2F-1 infection. Overexpression of a truncated E2F-1 protein that lacks the transactivation domain failed to up-regulate PUMA promoter, suggesting that PUMA may be a transcriptional target of E2F-1. E2F-1-induced cancer cell apoptosis was accompanied by Bax translocation from the cytosol to mitochondria and the induction of caspase-9 activity, suggesting that E2F-1-induced apoptosis is mediated by PUMA through the cytochrome C/Apaf-1-dependent pathway.
Conclusion: Our studies strongly demonstrated that E2F-1 induces melanoma cell apoptosis via PUMA up-regulation and Bax translocation. The signaling pathways provided here will further enhance insights on the mechanisms of E2F-1-induced cancer cell apoptosis as a strategy for cancer therapy.
Figures


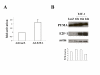

Similar articles
-
Induction of apoptosis signal-regulating Kinase 1 by E2F-1 may not be essential for E2F-1-mediated apoptosis in melanoma cells.Tumour Biol. 2007;28(2):111-22. doi: 10.1159/000099370. Epub 2007 Feb 8. Tumour Biol. 2007. PMID: 17287612
-
Apaf-1 is a mediator of E2F-1-induced apoptosis.J Biol Chem. 2002 Oct 18;277(42):39760-8. doi: 10.1074/jbc.M200805200. Epub 2002 Jul 30. J Biol Chem. 2002. PMID: 12149244
-
p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation.J Biol Chem. 2004 Feb 27;279(9):8076-83. doi: 10.1074/jbc.M307469200. Epub 2003 Nov 21. J Biol Chem. 2004. PMID: 14634023
-
Tumor-specific adenovirus-mediated PUMA gene transfer using the survivin promoter enhances radiosensitivity of breast cancer cells in vitro and in vivo.Breast Cancer Res Treat. 2009 Sep;117(1):45-54. doi: 10.1007/s10549-008-0163-6. Epub 2008 Sep 13. Breast Cancer Res Treat. 2009. PMID: 18791823
-
Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells.Biochem Pharmacol. 2008 May 15;75(10):2020-33. doi: 10.1016/j.bcp.2008.02.023. Epub 2008 Feb 29. Biochem Pharmacol. 2008. PMID: 18377871 Free PMC article.
Cited by
-
Melanoma biomarkers: current status and vision for the future.Nat Clin Pract Oncol. 2009 Feb;6(2):105-17. doi: 10.1038/ncponc1296. Epub 2008 Dec 23. Nat Clin Pract Oncol. 2009. PMID: 19107110 Review.
-
The pro-apoptotic activity of Drosophila Rbf1 involves dE2F2-dependent downregulation of diap1 and buffy mRNA.Cell Death Dis. 2014 Sep 4;5(9):e1405. doi: 10.1038/cddis.2014.372. Cell Death Dis. 2014. PMID: 25188515 Free PMC article.
-
PUMA, a potent killer with or without p53.Oncogene. 2008 Dec;27 Suppl 1(Suppl 1):S71-83. doi: 10.1038/onc.2009.45. Oncogene. 2008. PMID: 19641508 Free PMC article. Review.
-
pRb/E2F-1-mediated caspase-dependent induction of Noxa amplifies the apoptotic effects of the Bcl-2/Bcl-xL inhibitor ABT-737.Cell Death Differ. 2013 May;20(5):755-64. doi: 10.1038/cdd.2013.6. Epub 2013 Feb 22. Cell Death Differ. 2013. PMID: 23429261 Free PMC article.
-
BH3-based fusion artificial peptide induces apoptosis and targets human colon cancer.Mol Ther. 2009 Sep;17(9):1509-16. doi: 10.1038/mt.2009.43. Epub 2009 Apr 7. Mol Ther. 2009. PMID: 19352325 Free PMC article.
References
-
- Bell L, Ryan KM. Life and death decisions by E2F-1. Cell Death Differ. 2004;11:137–142. - PubMed
-
- DeGregori J. The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochim Biophys Acta. 2002;1602:131–150. - PubMed
-
- Dong YB, Yang HL, Elliott MJ, Liu TJ, Stilwell A, Atienza C, Jr, McMasters KM. Adenovirus-mediated E2F-1 gene transfer efficiently induces apoptosis in melanoma cells. Cancer. 1999;86:2021–2033. - PubMed
-
- Yang HL, Dong YB, Elliott MJ, Liu TJ, McMasters KM. Caspase activation and changes in Bcl-2 family member protein expression associated with E2F-1-mediated apoptosis in human esophageal cancer cells. Clin Cancer Res. 2000;6:1579–1589. - PubMed
-
- Dong YB, Yang HL, McMasters KM. E2F-1 overexpression sensitizes colorectal cancer cells to camptothecin. Cancer Gene Ther. 2003;10:168–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

