Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes
- PMID: 17263893
- PMCID: PMC1797809
- DOI: 10.1186/1471-2180-7-9
Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes
Abstract
Background: To be transmitted by its mosquito vector, dengue virus (DENV) must infect midgut epithelial cells, replicate and disseminate into the hemocoel, and finally infect the salivary glands, which is essential for transmission. The extrinsic incubation period (EIP) is very relevant epidemiologically and is the time required from the ingestion of virus until it can be transmitted to the next vertebrate host. The EIP is conditioned by the kinetics and tropisms of virus replication in its vector. Here we document the virogenesis of DENV-2 in newly-colonized Aedes aegypti mosquitoes from Chetumal, Mexico in order to understand better the effect of vector-virus interactions on dengue transmission.
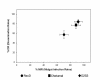
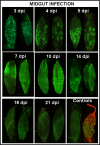
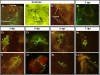
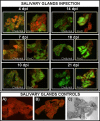
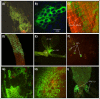
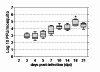
Results: After ingestion of DENV-2, midgut infections in Chetumal mosquitoes were characterized by a peak in virus titers between 7 and 10 days post-infection (dpi). The amount of viral antigen and viral titers in the midgut then declined, but viral RNA levels remained stable. The presence of DENV-2 antigen in the trachea was positively correlated with virus dissemination from the midgut. DENV-2 antigen was found in salivary gland tissue in more than a third of mosquitoes at 4 dpi. Unlike in the midgut, the amount of viral antigen (as well as the percent of infected salivary glands) increased with time. DENV-2 antigen also accumulated and increased in neural tissue throughout the EIP. DENV-2 antigen was detected in multiple tissues of the vector, but unlike some other arboviruses, was not detected in muscle.
Conclusion: Our results suggest that the EIP of DENV-2 in its vector may be shorter that the previously reported and that the tracheal system may facilitate DENV-2 dissemination from the midgut. Mosquito organs (e.g. midgut, neural tissue, and salivary glands) differed in their response to DENV-2 infection.
Figures
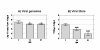

References
-
- Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

