Functionally and spatially distinct modes of munc18-syntaxin 1 interaction
- PMID: 17264080
- PMCID: PMC1891423
- DOI: 10.1074/jbc.M700227200
Functionally and spatially distinct modes of munc18-syntaxin 1 interaction
Abstract
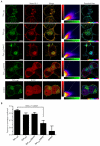
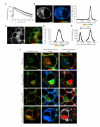
Eukaryotic membrane trafficking is a conserved process under tight temporal and spatial regulation in which the fusion of membranes is driven by the formation of the ternary SNARE complex. Syntaxin 1a, a core component of the exocytic SNARE complex in neurons and neuroendocrine cells, is regulated directly by munc18-1, its cognate Sec1p/munc18 (SM) protein. SM proteins show remarkable structural conservation throughout evolution, indicating a common binding mechanism and function. However, SM proteins possess disparate binding mechanisms and regulatory effects with munc18-1, the major brain isoform, classed as atypical in both its binding specificity and its mode. We now show that munc18-1 interacts with syntaxin 1a through two mechanistically distinct modes of binding, both in vitro and in living cells, in contrast to current models. Furthermore, these functionally divergent interactions occur at distinct cellular locations. These findings provide a molecular explanation for the multiple, spatially distinct roles of munc18-1.
Figures


References
-
- Burgoyne RD, Morgan A. Physiol Rev. 2003;83(2):581–632. - PubMed
-
- Rizo J, Sudhof TC. Nat Rev Neurosci. 2002;3(8):641–653. - PubMed
-
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Nature. 1998;395(6700):347–353. - PubMed
-
- Harrison SD, Broadie K, van de Goor J, Rubin GM. Neuron. 1994;13(3):555–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

