Alternating-electric-field-enhanced reversible switching of DNA nanocontainers with pH
- PMID: 17264114
- PMCID: PMC1865044
- DOI: 10.1093/nar/gkl1161
Alternating-electric-field-enhanced reversible switching of DNA nanocontainers with pH
Abstract
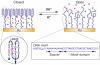
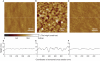
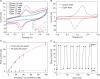
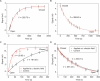
Macroscopically realizable applications of DNA-based molecular devices require individual molecules to cooperate with each other. However, molecular crowding usually introduces disorder to the system, thus jeopardizing the molecular cooperation and slowing down their functional performance dramatically. A challenge remaining in this field is to obtain both smarter response and better cooperation simultaneously. Here, we report a swift-switching DNA nanodevice that is enhanced by an alternating electric field. The device, self-assembled from folded four-stranded DNA motifs, can robustly switch between closed and open states in smart response to pH stimulus, of which the closed state forms a nanometer-height container that is impermeable to small molecules. This character was used to directly and non-specifically catch and release small molecules emulating mechanical hand in a controllable way. The alternating electric field was used to accelerate molecular cooperative motion during the device switching, which in turn shortened the closing time remarkably to thirty seconds.
Figures
Similar articles
-
Stimuli-responsive controlled-release system using quadruplex DNA-capped silica nanocontainers.Nucleic Acids Res. 2011 Mar;39(4):1638-44. doi: 10.1093/nar/gkq893. Epub 2010 Oct 21. Nucleic Acids Res. 2011. PMID: 20965972 Free PMC article.
-
"Nano-oddities": unusual nucleic acid assemblies for DNA-based nanostructures and nanodevices.Acc Chem Res. 2014 Jun 17;47(6):1836-44. doi: 10.1021/ar500063x. Epub 2014 May 28. Acc Chem Res. 2014. PMID: 24871086
-
An electrochemically actuated reversible DNA switch.Nano Lett. 2010 Apr 14;10(4):1393-7. doi: 10.1021/nl100169p. Nano Lett. 2010. PMID: 20218663
-
Nucleic Acid i-Motif Structures in Analytical Chemistry.Crit Rev Anal Chem. 2016 Sep 2;46(5):443-54. doi: 10.1080/10408347.2016.1143347. Epub 2016 Mar 3. Crit Rev Anal Chem. 2016. PMID: 26939549 Review.
-
Target-responsive structural switching for nucleic acid-based sensors.Acc Chem Res. 2010 May 18;43(5):631-41. doi: 10.1021/ar900245u. Acc Chem Res. 2010. PMID: 20222738 Review.
Cited by
-
Epigenetic modification, dehydration, and molecular crowding effects on the thermodynamics of i-motif structure formation from C-rich DNA.Biochemistry. 2014 Mar 18;53(10):1586-94. doi: 10.1021/bi401523b. Epub 2014 Mar 6. Biochemistry. 2014. PMID: 24564458 Free PMC article.
-
Reversible Regulation of Catalytic Activity of Gold Nanoparticles with DNA Nanomachines.Sci Rep. 2015 Sep 23;5:14402. doi: 10.1038/srep14402. Sci Rep. 2015. PMID: 26395968 Free PMC article.
-
General Strategy to Introduce pH-Induced Allostery in DNA-Based Receptors to Achieve Controlled Release of Ligands.Nano Lett. 2015 Jul 8;15(7):4467-71. doi: 10.1021/acs.nanolett.5b00852. Epub 2015 Jun 10. Nano Lett. 2015. PMID: 26053894 Free PMC article.
-
Dual restriction enzyme digest of cationic-gold-coated DNA scaffolds.Int J Nanomedicine. 2007;2(4):821-5. Int J Nanomedicine. 2007. PMID: 18203449 Free PMC article.
-
Ferrocene-Containing DNA Monolayers: Influence of Electrostatics on the Electron Transfer Dynamics.Langmuir. 2021 Mar 23;37(11):3359-3369. doi: 10.1021/acs.langmuir.0c03485. Epub 2021 Mar 11. Langmuir. 2021. PMID: 33705153 Free PMC article.
References
-
- Balzani V, Credi A, Venturi M. Molecular Devices and Machines – A Journey into Nano World. Weinheim, Germany: Wiley-VCH; 2003.
-
- Seeman NC. DNA in a material world. Nature. 2003;421:427–431. - PubMed
-
- Mao C, Sun W, Shen Z, Seeman NC. A nanomechanical device based on the B-Z transition of DNA. Nature. 1999;397:144–146. - PubMed
-
- Yurke B, Turberfield AJ, Mills AP, Jr., Neumann JL. A DNA-fuelled molecular machine made of DNA. Nature. 2000;406:605–608. - PubMed
-
- Yan H, Zhang X, Shen Z, Seeman NC. A robust DNA mechanical device controlled by hybridization topology. Nature. 2002;415:62–65. - PubMed

