Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511
- PMID: 17264295
- PMCID: PMC1885493
- DOI: 10.1182/blood-2006-09-045351
Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511
Abstract
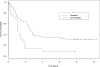
CALGB 9511 used pegaspargase (PEG-ASP) in lieu of the native enzyme. The aim was to compare differences in overall survival (OS) and disease-free survival (DFS) between patients who did and did not achieve asparagine depletion, defined by enzyme levels greater than 0.03 U/mL plasma for 14 consecutive days after at least 1 of 4 planned PEG-ASP administrations. Samples were available from 85 eligible patients. On univariate analyses, the 22 patients who did not achieve asparagine depletion had inferior OS (P = .002; hazard ratio [HR] = 2.37; 95% CI = 1.38-4.09) and DFS (P = .012; HR = 2.21; 95% CI = 1.19-4.13). After adjusting for age, performance status, leukocyte count, and karyotype in a proportional hazards model, both the OS and DFS HRs decreased to 1.8 (P = .056; 95% CI = 1.0-3.2 and P = .084; 95% CI = 0.9-3.6, respectively). We conclude that effective asparagine depletion with PEG-ASP is feasible as part of an intensive multiagent therapeutic regimen in adult acute lymphoblastic leukemia and appears associated with improved outcomes.
Figures
References
-
- Keating MJ, Holmes R, Lerner S, Ho DH. L-asparaginase and PEG asparaginase— past, present, and future. Leuk Lymphoma. 1993;10(suppl):153–157. - PubMed
-
- Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993;11:1780–1786. - PubMed
-
- Asselin BL, Ryan D, Frantz CN, et al. In vitro and in vivo killing of acute lymphoblastic leukemia cells by L-asparaginase. Cancer Res. 1989;49:4363–4368. - PubMed
-
- Ettinger LJ, Kurtzberg J, Voute PA, Jurgens H, Halpern SL. An open-label, multicenter study of polyethylene glycol-L-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer. 1995;75:1176–1181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA047577/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- CA101140/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- CA02599/CA/NCI NIH HHS/United States
- CA47577/CA/NCI NIH HHS/United States
- CA41287/CA/NCI NIH HHS/United States
- U10 CA035279/CA/NCI NIH HHS/United States
- CA03927/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- CA35279/CA/NCI NIH HHS/United States
- U10 CA101140/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- CA77658/CA/NCI NIH HHS/United States
- CA31983/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA003927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources