Hmgcr in the corpus allatum controls sexual dimorphism of locomotor activity and body size via the insulin pathway in Drosophila
- PMID: 17264888
- PMCID: PMC1779623
- DOI: 10.1371/journal.pone.0000187
Hmgcr in the corpus allatum controls sexual dimorphism of locomotor activity and body size via the insulin pathway in Drosophila
Abstract
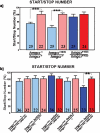
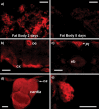
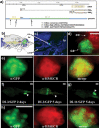
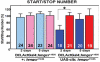
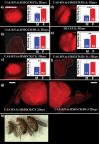
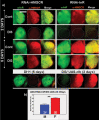
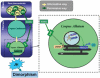
The insulin signaling pathway has been implicated in several physiological and developmental processes. In mammals, it controls expression of 3-Hydroxy-3-Methylglutaryl CoA Reductase (HMGCR), a key enzyme in cholesterol biosynthesis. In insects, which can not synthesize cholesterol de novo, the HMGCR is implicated in the biosynthesis of juvenile hormone (JH). However, the link between the insulin pathway and JH has not been established. In Drosophila, mutations in the insulin receptor (InR) decrease the rate of JH synthesis. It is also known that both the insulin pathway and JH play a role in the control of sexual dimorphism in locomotor activity. In studies here, to demonstrate that the insulin pathway and HMGCR are functionally linked in Drosophila, we first show that hmgcr mutation also disrupts the sexual dimorphism. Similarly to the InR, HMGCR is expressed in the corpus allatum (ca), which is the gland where JH biosynthesis occurs. Two p[hmgcr-GAL4] lines were therefore generated where RNAi was targeted specifically against the HMGCR or the InR in the ca. We found that RNAi-HMGCR blocked HMGCR expression, while the RNAi-InR blocked both InR and HMGCR expression. Each RNAi caused disruption of sexual dimorphism and produced dwarf flies at specific rearing temperatures. These results provide evidence: (i) that HMGCR expression is controlled by the InR and (ii) that InR and HMGCR specifically in the ca, are involved in the control of body size and sexual dimorphism of locomotor activity.
Conflict of interest statement
Figures

References
-
- Takahashi Y, Tobe K, Kadowaki H, Katsumata D, Fukushima Y, et al. Roles of insulin receptor substrate-1 and Shc on insulin-like growth factor I receptor signaling in early passages of cultured human fibroblasts. Endocrinology. 1997;138:741–750. - PubMed
-
- Butler AA, Le Roith D. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol. 2001;63:141–164. - PubMed
-
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. - PubMed
-
- Foufelle F, Ferre P. [Regulation of carbohydrate metabolism by insulin: role of transcription factor SREBP-1c in the hepatic transcriptional effects of the hormone]. J Soc Biol. 2001;195:243–248. - PubMed
-
- Penhos JC, Wu CH, Lemberg A, Daunas J, Brodoff B, et al. The effect of insulin on the metabolism of lipids and on urea formation by the perfused rat liver. Metabolism. 1968;17:246–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

