Development of synthetic membrane transporters for anions
- PMID: 17264935
- PMCID: PMC2854546
- DOI: 10.1039/b512651g
Development of synthetic membrane transporters for anions
Abstract
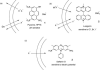
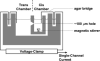
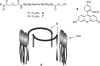
The development of low molecular weight anion transporters is an emerging topic in supramolecular chemistry. The major focus of this tutorial review is on synthetic chloride transport systems that operate in vesicle and cell membranes. The transporters alter transmembrane concentration gradients, and thus they have applications as reagents for cell biology research and as potential chemotherapeutic agents. The molecular designs include monomolecular channels, self-assembled channels and mobile carriers. Also discussed are the experimental assays that measure transport rates across model bilayer membranes.
Figures
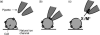

References
-
- Sessler JL, Gale PA, Cho W-S. Anion Receptor Chemistry. Royal Society of Chemistry; Cambridge: 2006.
-
- Moyer BA, Singh R, editors. ACS Symp. Ser. Kluwer; New York: 2004. Fundamentals and Applications of Anion Separations.
-
- Berkowitz ML, Bostick DL, Pandit S. Chem. Rev. 2006;106:1527. - PubMed
-
- Eisenman G, Horn RJ. J. Membr. Biol. 1983;76:197. - PubMed
-
- Gurtovenko AA, Vattulainen L. J. Am. Chem. Soc. 2005;127:17570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

