On the substrate specificity of dehydration by lacticin 481 synthetase
- PMID: 17266311
- PMCID: PMC2517113
- DOI: 10.1021/ja067672v
On the substrate specificity of dehydration by lacticin 481 synthetase
Abstract
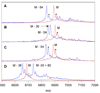
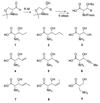
Dehydroamino acids are valuable building blocks that are a challenge to incorporate synthetically into unprotected peptides. Lantibiotic synthetases possess dehydration activity that converts Ser and Thr residues in their peptide substrates into dehydroalanine and dehydrobutyrine residues, respectively. We show here that lacticin 481 synthetase can convert the Thr analogs (R)-3-EtSer, (R)-3-vinylSer, (R)-3-ethynylSer, and (R)-3-[(E)-propenyl]Ser into the corresponding dehydro amino acids when incorporated into its peptide substrate. This relaxed substrate specificity holds promise for using the enzyme for synthetic purposes and for lantibiotic engineering. On the other hand, (R)-3-PrSer, (R)-3-iPrSer, and allo-Thr are not substrates for the enzyme.
Figures

Similar articles
-
Lacticin 481: in vitro reconstitution of lantibiotic synthetase activity.Science. 2004 Jan 30;303(5658):679-81. doi: 10.1126/science.1092600. Science. 2004. PMID: 14752162
-
The dehydratase activity of lacticin 481 synthetase is highly processive.J Am Chem Soc. 2006 Feb 8;128(5):1420-1. doi: 10.1021/ja057203d. J Am Chem Soc. 2006. PMID: 16448091 Free PMC article.
-
Mechanistic investigations of the dehydration reaction of lacticin 481 synthetase using site-directed mutagenesis.Biochemistry. 2007 May 22;46(20):5991-6000. doi: 10.1021/bi602663x. Epub 2007 Apr 25. Biochemistry. 2007. PMID: 17455908 Free PMC article.
-
Approaches to the discovery of new antibacterial agents based on bacteriocins.Biochem Cell Biol. 2008 Apr;86(2):116-23. doi: 10.1139/O07-153. Biochem Cell Biol. 2008. PMID: 18443625 Review.
-
Rings, radicals, and regeneration: the early years of a bioorganic laboratory.J Org Chem. 2006 Dec 22;71(26):9561-71. doi: 10.1021/jo0614240. J Org Chem. 2006. PMID: 17168571 Free PMC article. Review.
Cited by
-
De novo synthesis of D- and L-fucosamine containing disaccharides.Beilstein J Org Chem. 2013;9:332-41. doi: 10.3762/bjoc.9.38. Epub 2013 Feb 14. Beilstein J Org Chem. 2013. PMID: 23503315 Free PMC article.
-
Use of lantibiotic synthetases for the preparation of bioactive constrained peptides.Bioorg Med Chem Lett. 2008 May 15;18(10):3025-8. doi: 10.1016/j.bmcl.2008.01.062. Epub 2008 Jan 19. Bioorg Med Chem Lett. 2008. PMID: 18294843 Free PMC article.
-
The presence of modifiable residues in the core peptide part of precursor nisin is not crucial for precursor nisin interactions with NisB- and NisC.PLoS One. 2013 Sep 9;8(9):e74890. doi: 10.1371/journal.pone.0074890. eCollection 2013. PLoS One. 2013. PMID: 24040355 Free PMC article.
-
On the regioselectivity of thioether formation by lacticin 481 synthetase.Org Lett. 2007 Aug 16;9(17):3343-6. doi: 10.1021/ol071301h. Epub 2007 Jul 25. Org Lett. 2007. PMID: 17650008 Free PMC article.
-
Feasability of Introducing a Thioether Ring in Vasopressin by nisBTC Co-expression in Lactococcus lactis.Front Microbiol. 2019 Jul 2;10:1508. doi: 10.3389/fmicb.2019.01508. eCollection 2019. Front Microbiol. 2019. PMID: 31333616 Free PMC article.
References
-
-
For selected examples, seeBodanszky M, Scozzie JA, Muramatsu I. J. Antibiot. 1970;23:9–12.Berdy J. Adv. Appl. Microbiol. 1974;18:309–406.Tori K, Tokura K, Okabe K, Ebata M, Otsuka H, Lukacs G. Tetrahedron Lett. 1976;3:185–188.Pascard C, Ducruix A, Lunel J, Prangé T. J. Am. Chem. Soc. 1977;99:6418–6423.Pearce CJ, Rinehart KL., Jr. J. Am. Chem. Soc. 1979;101:5069–5070.Pedras MSC, Taylor JT, Nakashima TT. J. Org. Chem. 1993;58:4778–4780.Hamann MT, Scheuer PJ. J. Am. Chem. Soc. 1993;115:5825–5826.Lau RC, Rinehart KL., Jr. J. Antibiot. 1994;47:1466–1472.For selected synthetic studies, seeSnider BB, Zeng H. J. Org. Chem. 2003;68:545–563.Nicolaou KC, Zak M, Safina BS, Lee SH, Estrada AA. Angew Chem Int Ed Engl. 2004;43:5092–5097.Ley SV, Priour A, Heusser C. Org. Lett. 2002;4:711–714.
-
-
- Palmer DE, Pattaroni C, Nunami K, Chadha RK, Goodman M, Wakamiya T, Fukase K, Horimoto S, Kitazawa M, Fujita H, Kubo A, Shiba T. J. Am. Chem. Soc. 1992;114:5634–5642.
- Lombardi A, D'Agostino B, Nastri F, D'Andrea LD, Filippelli A, Falciani M, Rossi F, Pavone V. Bioorg. Med. Chem. Lett. 1998;8:1153–1156. - PubMed
- Murkin AS, Tanner ME. J. Org. Chem. 2002;67:8389–8394. - PubMed
- Li B, Yu J-PJ, Brunzelle JS, Moll GN, van der Donk WA, Nair SK. Science. 2006;311:1464–1467. - PubMed
-
- Schmidt U, Lieberknecht A, Wild J. Synthesis. 1988:159–172.
- Bonauer C, Walenczyk T, König B. Synthesis. 2006:1–20.
-
- Chatterjee C, Paul M, Xie L, van der Donk WA. Chem. Rev. 2005;105:633–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

