Targeting purine and pyrimidine metabolism in human apicomplexan parasites
- PMID: 17266529
- PMCID: PMC2720675
- DOI: 10.2174/138945007779315524
Targeting purine and pyrimidine metabolism in human apicomplexan parasites
Abstract
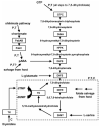
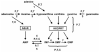
Synthesis de novo, acquisition by salvage and interconversion of purines and pyrimidines represent the fundamental requirements for their eventual assembly into nucleic acids as nucleotides and the deployment of their derivatives in other biochemical pathways. A small number of drugs targeted to nucleotide metabolism, by virtue of their effect on folate biosynthesis and recycling, have been successfully used against apicomplexan parasites such as Plasmodium and Toxoplasma for many years, although resistance is now a major problem in the prevention and treatment of malaria. Many targets not involving folate metabolism have also been explored at the experimental level. However, the unravelling of the genome sequences of these eukaryotic unicellular organisms, together with increasingly sophisticated molecular analyses, opens up possibilities of introducing new drugs that could interfere with these processes. This review examines the status of established drugs of this type and the potential for further exploiting the vulnerability of apicomplexan human pathogens to inhibition of this key area of metabolism.
Figures

Similar articles
-
New drugs and drug targets for human diseases caused by apicomplexan parasites.Curr Drug Targets. 2007 Jan;8(1):1-2. doi: 10.2174/138945007779315588. Curr Drug Targets. 2007. PMID: 17266526 No abstract available.
-
Toxoplasma gondii: the model apicomplexan.Int J Parasitol. 2004 Mar 9;34(3):423-32. doi: 10.1016/j.ijpara.2003.12.009. Int J Parasitol. 2004. PMID: 15003501 Free PMC article. Review.
-
Purine and pyrimidine metabolism in Leishmania.Adv Exp Med Biol. 2008;625:141-54. doi: 10.1007/978-0-387-77570-8_12. Adv Exp Med Biol. 2008. PMID: 18365665 Review.
-
Purines and pyrimidines in malarial parasites.Blood Cells. 1990;16(2-3):467-84; discussion 485-98. Blood Cells. 1990. PMID: 2257323 Review.
-
Biosynthetic pathways of plastid-derived organelles as potential drug targets against parasitic apicomplexa.Curr Drug Targets Immune Endocr Metabol Disord. 2003 Jun;3(2):99-109. doi: 10.2174/1568008033340261. Curr Drug Targets Immune Endocr Metabol Disord. 2003. PMID: 12769782 Review.
Cited by
-
Acyclic phosph(on)ate inhibitors of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase.Bioorg Med Chem. 2013 Sep 1;21(17):5629-46. doi: 10.1016/j.bmc.2013.02.016. Epub 2013 Mar 5. Bioorg Med Chem. 2013. PMID: 23810424 Free PMC article.
-
Genomics of apicomplexan parasites.Crit Rev Biochem Mol Biol. 2017 Jun;52(3):254-273. doi: 10.1080/10409238.2017.1290043. Epub 2017 Feb 22. Crit Rev Biochem Mol Biol. 2017. PMID: 28276701 Free PMC article. Review.
-
Comparison of Proteins Secreted into Extracellular Space of Pathogenic and Non-pathogenic Acanthamoeba castellanii.Korean J Parasitol. 2018 Dec;56(6):553-558. doi: 10.3347/kjp.2018.56.6.553. Epub 2018 Dec 31. Korean J Parasitol. 2018. PMID: 30630275 Free PMC article.
-
Reexamining Chronic Toxoplasma gondii Infection: Surprising Activity for a "Dormant" Parasite.Curr Clin Microbiol Rep. 2016 Dec;3(4):175-185. doi: 10.1007/s40588-016-0045-3. Epub 2016 Oct 4. Curr Clin Microbiol Rep. 2016. PMID: 28191447 Free PMC article.
-
Drug-resistant malaria - an insight.FEBS J. 2007 Sep;274(18):4688-98. doi: 10.1111/j.1742-4658.2007.05999.x. FEBS J. 2007. PMID: 17824955 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
