Expression of costimulatory molecules in the bovine corpus luteum
- PMID: 17266770
- PMCID: PMC1800853
- DOI: 10.1186/1477-7827-5-5
Expression of costimulatory molecules in the bovine corpus luteum
Abstract
Background: Bovine luteal parenchymal cells express class II major histocompatibility complex (MHC) molecules and stimulate class II MHC-dependent activation of T cells in vitro. The ability of a class II MHC-expressing cell type to elicit a response from T cells in vivo is also dependent on expression of costimulatory molecules by the antigen presenting cell and delivery of a costimulatory signal to the T cell. Whether bovine luteal parenchymal cells express costimulatory molecules and can deliver the costimulatory signal is currently unknown.
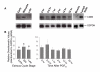
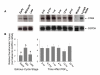
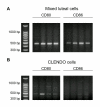
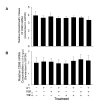
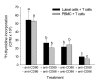
Methods: Bovine luteal tissue was collected during the early (day 5; day of estrus = day 0), mid (day 11-12), or late (day 18) luteal phase of the estrous cycle, and at 0, 0.5, 1, 4, 12 or 24 hours following administration of PGF2alpha to cows on day 10 of the estrous cycle. Northern analysis was used to measure CD80 or CD86 mRNA concentrations in luteal tissue samples. Mixed luteal parenchymal cell cultures and purified luteal endothelial cell cultures were prepared, and real-time RT-PCR was used to examine the presence of CD80 and CD86 mRNA in each culture type. Monoclonal antibodies to CD80 and CD86 were added to a mixed luteal parenchymal cell-T cell co-culture in vitro T cell proliferation assay to assess the functional significance of costimulatory molecules on activation of T lymphocytes by luteal parenchymal cells.
Results: Northern analysis revealed CD80 and CD86 mRNAs in luteal tissue, with greatest steady-state concentrations at midcycle. CD80 and CD86 mRNAs were detected in mixed luteal parenchymal cell cultures, but only slight amounts of CD80 (and not CD86) mRNA were detected in cultures of luteal endothelial cells. Luteinizing hormone, PGF2alpha and TNF-alpha were without effect on concentrations of CD80 or CD86 mRNA in mixed luteal parenchymal cells cultures. Anti-CD80 or anti-CD86 monoclonal antibodies inhibited T cell proliferation in the in vitro T cell proliferation assay.
Conclusion: It can be concluded from this study that parenchymal cells within the bovine CL express functional costimulatory molecules that facilitate interactions between with T cells, and these components of the antigen presentation pathway are expressed maximally in the midcycle CL.
Figures
Similar articles
-
Presence and regulation of messenger ribonucleic acids encoding components of the class II major histocompatibility complex-associated antigen processing pathway in the bovine corpus luteum.Reproduction. 2006 Apr;131(4):689-98. doi: 10.1530/rep.1.00906. Reproduction. 2006. PMID: 16595720
-
The class II major histocompatibility complex molecule BoLA-DR is expressed by endothelial cells of the bovine corpus luteum.Reproduction. 2007 May;133(5):991-1003. doi: 10.1530/REP-06-0362. Reproduction. 2007. PMID: 17616728
-
[Role of MG132, a proteasome inhibitor, in inducing expression of costimulatory molecules CD86 in leukemic cells and its effect on allogeneic mixed lymphocyte reaction].Zhonghua Yi Xue Za Zhi. 2007 Mar 13;87(10):710-3. Zhonghua Yi Xue Za Zhi. 2007. PMID: 17553313 Chinese.
-
The role of major histocompatibility complex molecules in luteal function.Reprod Biol Endocrinol. 2003 Nov 10;1:93. doi: 10.1186/1477-7827-1-93. Reprod Biol Endocrinol. 2003. PMID: 14613531 Free PMC article. Review. No abstract available.
-
Signaling through CD80: an approach for treating lymphomas.Expert Opin Ther Targets. 2008 Aug;12(8):969-79. doi: 10.1517/14728222.12.8.969. Expert Opin Ther Targets. 2008. PMID: 18620519 Review.
Cited by
-
Form of Supplemental Selenium Affects the Expression of mRNA Transcripts Encoding Selenoproteins, and Proteins Regulating Cholesterol Uptake, in the Corpus Luteum of Grazing Beef Cows.Animals (Basel). 2022 Jan 27;12(3):313. doi: 10.3390/ani12030313. Animals (Basel). 2022. PMID: 35158637 Free PMC article.
-
History, insights, and future perspectives on studies into luteal function in cattle.J Anim Sci. 2022 Jul 1;100(7):skac143. doi: 10.1093/jas/skac143. J Anim Sci. 2022. PMID: 35772753 Free PMC article. Review.
-
Mechanisms of angioregression of the corpus luteum.Front Physiol. 2023 Sep 28;14:1254943. doi: 10.3389/fphys.2023.1254943. eCollection 2023. Front Physiol. 2023. PMID: 37841308 Free PMC article. Review.
-
Molecular characterization and expression of DERL1 in bovine ovarian follicles and corpora lutea.Reprod Biol Endocrinol. 2010 Aug 3;8:94. doi: 10.1186/1477-7827-8-94. Reprod Biol Endocrinol. 2010. PMID: 20682045 Free PMC article.
References
-
- Lawler DF, Hopkins J, Watson DE. Immune cell populations in the equine corpus luteum throughout the oestrous cycle and early pregnancy: an immunohistochemical and flow cytometric study. J Reprod Fert. 1999;117:281–290. - PubMed
-
- Penny LA, Armstrong D, Bramley TA, Webb R, Collins RA, Watson ED. Immune cells and cytokine production in the bovine corpus luteum throughout the oestrous cycle and after induced luteolysis. J Reprod Fert. 1999;115:87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

