Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury
- PMID: 17267172
- PMCID: PMC5021535
- DOI: 10.1016/j.bbi.2006.10.017
Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury
Abstract
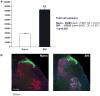
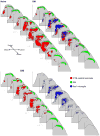
The involvement of glia, and glia-neuronal signalling in enhancing nociceptive transmission has become an area of intense scientific interest. In particular, a role has emerged for activated microglia in the development and maintenance of neuropathic pain following peripheral nerve injury. Following activation, spinal microglia proliferate and release many substances which are capable of modulating neuronal excitability within the spinal cord. Here, we the investigated the response of spinal microglia to a unilateral spared nerve injury (SNI) in terms of the quantitative increase in cell number and the spatial distribution of the increase. Design-based stereological techniques were combined with iba-1 immunohistochemistry to estimate the total number of microglia in the spinal dorsal horn in naïve and peripheral nerve-injured adult rats. In addition, by mapping the central terminals of hindlimb nerves, the somatotopic distribution of the microglial response was mapped. Following SNI there was a marked increase in the number of spinal microglia: The total number of microglia (mean+/-SD) in the dorsal horn sciatic territory of the naïve rat was estimated to be 28,591+/-2715. Following SNI the number of microglia was 82,034+/-8828. While the pattern of microglial activation generally followed somatotopic boundaries, with the majority of microglia within the territory occupied by peripherally axotomised primary afferents, some spread was seen into regions occupied by intact, 'spared' central projections of the sural nerve. This study provides a reproducible method of assaying spinal microglial dynamics following peripheral nerve injury both quantitatively and spatially.
Figures



References
-
- Coggeshall RE, Lekan HA, Doubell TP, Allchorne A, Woolf CJ. Central changes in primary afferent fibers following peripheral nerve lesions. Neuroscience. 1997;77(4):1115–1122. - PubMed
-
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79(2):163–175. - PubMed
-
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157(2):289–304. - PubMed
-
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

