Lysine propionylation and butyrylation are novel post-translational modifications in histones
- PMID: 17267393
- PMCID: PMC2911958
- DOI: 10.1074/mcp.M700021-MCP200
Lysine propionylation and butyrylation are novel post-translational modifications in histones
Abstract
The positively charged lysine residue plays an important role in protein folding and functions. Neutralization of the charge often has a profound impact on the substrate proteins. Accordingly all the known post-translational modifications at lysine have pivotal roles in cell physiology and pathology. Here we report the discovery of two novel, in vivo lysine modifications in histones, lysine propionylation and butyrylation. We confirmed, by in vitro labeling and peptide mapping by mass spectrometry, that two previously known acetyltransferases, p300 and CREB-binding protein, could catalyze lysine propionylation and lysine butyrylation in histones. Finally p300 and CREB-binding protein could carry out autopropionylation and autobutyrylation in vitro. Taken together, our results conclusively establish that lysine propionylation and lysine butyrylation are novel post-translational modifications. Given the unique roles of propionyl-CoA and butyryl-CoA in energy metabolism and the significant structural changes induced by the modifications, the two modifications are likely to have important but distinct functions in the regulation of biological processes.
Figures


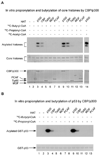
References
-
- Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 2005;44:7342–7372. - PubMed
-
- Walsh CT. Posttranslational Modifications of Proteins: Expanding Nature’s Inventory. Greenwood Village, CO: Roberts and Co. Publishers; 2006.
-
- Schweppe RE, Haydon CE, Lewis TS, Resing KA, Ahn NG. The characterization of protein post-translational modifications by mass spectrometry. Acc. Chem. Res. 2003;36:453–461. - PubMed
-
- Gu W, Shi XL, Roeder RG. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA098821/CA/NCI NIH HHS/United States
- CA098821/CA/NCI NIH HHS/United States
- CA104843/CA/NCI NIH HHS/United States
- CA107943/CA/NCI NIH HHS/United States
- R33 CA107943/CA/NCI NIH HHS/United States
- R01 GM031278/GM/NIGMS NIH HHS/United States
- R01 DK082664/DK/NIDDK NIH HHS/United States
- R01 CA126832/CA/NCI NIH HHS/United States
- R21 CA107943/CA/NCI NIH HHS/United States
- R01 CA104843/CA/NCI NIH HHS/United States
- GM31278/GM/NIGMS NIH HHS/United States
- AG025688/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

