Bluetongue virus entry into cells
- PMID: 17267479
- PMCID: PMC1900141
- DOI: 10.1128/JVI.02284-06
Bluetongue virus entry into cells
Erratum in
- J Virol. 2008 Feb;82(3):1626
Abstract
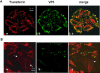
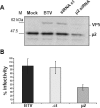
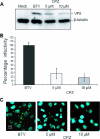
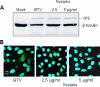
Bluetongue virus (BTV) is a member of the Orbivirus genus within the Reoviridae family. Like those of other members of the family, BTV particles are nonenveloped and contain two distinct capsids, namely, an outer capsid and an inner capsid or core. The two outer capsid proteins, VP2 and VP5, are involved in BTV entry into cells and in the delivery of the transcriptionally active core to the target cell cytoplasm. However, very little is known about the precise mechanism of BTV entry. In this report, using RNA interference, we demonstrate that inhibition of the clathrin-dependent endocytic pathway correlates with reduced BTV internalization and subsequent replication. Furthermore, by using the ATPase inhibitor bafilomycin A1, we show that exposure of the virus to acidic pH is required for productive infection. Moreover, microscopic analysis of cells incubated with BTV indicated that the virus is internalized into early endosomes, where separation of the outer capsid and inner core occurs. Together, our data indicate that BTV undergoes low-pH-induced penetration in early endosomes following clathrin-mediated endocytosis from the plasma membrane, supporting a stepwise model for BTV entry and penetration.
Figures



References
-
- Brezis, M., S. Rosen, P. Silva, K. Spokes, and F. H. Epstein. 1984. Polyene toxicity in renal medulla: injury mediated by transport activity. Science 224:66-68. - PubMed
-
- Chandran, K., and M. L. Nibert. 2003. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 11:374-382. - PubMed
-
- Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

