Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization
- PMID: 17267483
- PMCID: PMC1866094
- DOI: 10.1128/JVI.02626-06
Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization
Abstract
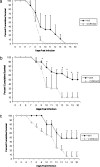
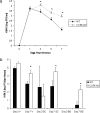
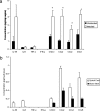
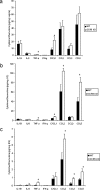
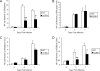
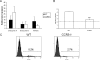
Following genital herpes simplex virus type 2 (HSV-2) exposure, NK cells and T cells are mobilized to sites of infection to control viral replication and spread. The present investigation sought to determine the role of the chemokine receptor CCR5 in this process. Mice deficient in CCR5 (CCR5-/-) displayed a significant reduction in cumulative survival following infection in comparison to wild-type, HSV-2-infected controls. Associated with decreased resistance to viral infection, CCR5-/- mice yielded significantly more virus and expressed higher levels of tumor necrosis factor alpha, CXCL1, CCL2, CCL3, and CCL5 in the vagina, spinal cord, and/or brain stem than did wild-type mice. Whereas there was no difference in absolute number of leukocytes (CD45high), CD4 T cells, or CD8 T cells residing in the draining lymph nodes, spleen, spinal cord, or brain stem comparing HSV-2-infected wild-type to CCR5-/- mice prior to or after infection, there were significantly more NK cells (NK1.1+ CD3-) residing in the brain stem and spleen of infected wild-type mice. Functionally, NK activity from cells isolated from the brain stem of HSV-2-infected wild-type mice was greater than that from HSV-2-infected CCR5-/- mice. In addition, antibody-mediated depletion of NK cells resulted in an increase in HSV-2 levels in the vaginal, spinal cord, and brain stem tissue of wild-type but not CCR5-/- mice. Collectively, the absence of CCR5 expression significantly impacts the ability of the host to control genital HSV-2 infection, inflammation, and spread associated with a specific reduction in NK cell expansion, infiltration, and activity in the nervous system.
Figures
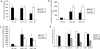
Similar articles
-
CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system.J Immunol. 2008 Jan 15;180(2):1098-106. doi: 10.4049/jimmunol.180.2.1098. J Immunol. 2008. PMID: 18178850 Free PMC article.
-
A role for the CCR5-CCL5 interaction in the preferential migration of HSV-2-specific effector cells to the vaginal mucosa upon nasal immunization.Mucosal Immunol. 2019 Nov;12(6):1391-1403. doi: 10.1038/s41385-019-0203-z. Epub 2019 Sep 24. Mucosal Immunol. 2019. PMID: 31551493
-
The lack of RNA-dependent protein kinase enhances susceptibility of mice to genital herpes simplex virus type 2 infection.Immunology. 2006 Aug;118(4):520-6. doi: 10.1111/j.1365-2567.2006.02403.x. Immunology. 2006. PMID: 16895559 Free PMC article.
-
Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1.J Gen Virol. 2006 Mar;87(Pt 3):489-499. doi: 10.1099/vir.0.81339-0. J Gen Virol. 2006. PMID: 16476970 Free PMC article.
-
Herpes simplex virus and the chemokines that mediate the inflammation.Curr Top Microbiol Immunol. 2006;303:47-65. doi: 10.1007/978-3-540-33397-5_3. Curr Top Microbiol Immunol. 2006. PMID: 16570856 Free PMC article. Review.
Cited by
-
Differential immune responses in pregnant patients recovered from COVID-19.Signal Transduct Target Ther. 2021 Jul 29;6(1):289. doi: 10.1038/s41392-021-00703-3. Signal Transduct Target Ther. 2021. PMID: 34326311 Free PMC article. Clinical Trial.
-
Immunoregulatory Functions of Interferons During Genital HSV-2 Infection.Front Immunol. 2021 Aug 18;12:724618. doi: 10.3389/fimmu.2021.724618. eCollection 2021. Front Immunol. 2021. PMID: 34484233 Free PMC article. Review.
-
Dual Function of Ccr5 during Langat Virus Encephalitis: Reduction in Neutrophil-Mediated Central Nervous System Inflammation and Increase in T Cell-Mediated Viral Clearance.J Immunol. 2016 Jun 1;196(11):4622-31. doi: 10.4049/jimmunol.1502452. Epub 2016 Apr 29. J Immunol. 2016. PMID: 27183602 Free PMC article.
-
Mucosal CCL28 Chemokine Improves Protection against Genital Herpes through Mobilization of Antiviral Effector Memory CCR10+CD44+ CD62L-CD8+ T Cells and Memory CCR10+B220+CD27+ B Cells into the Infected Vaginal Mucosa.J Immunol. 2023 Jul 1;211(1):118-129. doi: 10.4049/jimmunol.2300093. J Immunol. 2023. PMID: 37222480 Free PMC article.
-
Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection.J Exp Med. 2017 Apr 3;214(4):1153-1167. doi: 10.1084/jem.20160880. Epub 2017 Mar 6. J Exp Med. 2017. PMID: 28264883 Free PMC article.
References
-
- Aliberti, J., C. R. Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat. Immunol. 1:83-87. - PubMed
-
- Andres, P. G., P. L. Beck, E. Mizoguchi, A. Mizoguchi, A. K. Bhan, T. Dawson, W. A. Kuziel, N. Maeda, R. P. MacDermott, D. K. Podolsky, and H. C. Reinecker. 2000. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol. 164:6303-6312. - PubMed
-
- Armstrong, G. L., J. Schillinger, L. Markowitz, A. J. Nahmias, R. E. Johnson, G. M. McQuillan, and M. E. St. Louis. 2001. Incidence of herpes simplex virus type 2 infection in the United States. Am. J. Epidemiol. 153:912-920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

