Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor
- PMID: 17267500
- PMCID: PMC1866153
- DOI: 10.1128/JVI.02725-06
Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor
Abstract
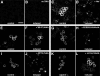
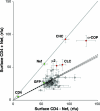
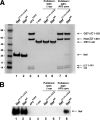
Nef, an accessory protein of human and simian immunodeficiency viruses, is a critical determinant of pathogenesis that promotes the progression from infection to AIDS. The pathogenic effects of Nef are in large part dependent on its ability to downregulate the macrophage and T-cell coreceptor, CD4. It has been proposed that Nef induces downregulation by linking the cytosolic tail of CD4 to components of the host-cell protein trafficking machinery. To identify these components, we developed a novel Nef-CD4 downregulation system in Drosophila melanogaster S2 cells. We found that human immunodeficiency virus type 1 (HIV-1) Nef downregulates human CD4 in S2 cells and that this process is subject to the same sequence requirements as in human cells. An RNA interference screen targeting protein trafficking genes in S2 cells revealed a requirement for clathrin and the clathrin-associated, plasma membrane-localized AP2 complex in the downregulation of CD4. The requirement for AP2 was confirmed in the human cell line HeLa. We also used a yeast three-hybrid system and glutathione S-transferase pull-down analyses to demonstrate a robust, direct interaction between HIV-1 Nef and AP2. This interaction requires a dileucine motif in Nef that is also essential for downregulation of CD4. Together, these results support a model in which HIV-1 Nef downregulates CD4 by promoting its accelerated endocytosis by a clathrin/AP2 pathway.
Figures





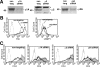

References
-
- Agaisse, H., L. S. Burrack, J. A. Philips, E. J. Rubin, N. Perrimon, and D. E. Higgins. 2005. Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science 309:1248-1251. - PubMed
-
- Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. - PubMed
-
- Benichou, S., M. Bomsel, M. Bodeus, H. Durand, M. Doute, F. Letourneur, J. Camonis, and R. Benarous. 1994. Physical interaction of the HIV-1 Nef protein with beta-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J. Biol. Chem. 269:30073-30076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

