Canine distemper virus infection requires cholesterol in the viral envelope
- PMID: 17267508
- PMCID: PMC1866149
- DOI: 10.1128/JVI.02647-06
Canine distemper virus infection requires cholesterol in the viral envelope
Abstract
Cholesterol is known to play an important role in stabilizing particular cellular membrane structures, so-called lipid or membrane rafts. For several viruses, a dependence on cholesterol for virus entry and/or morphogenesis has been shown. Using flow cytometry and fluorescence microscopy, we demonstrate that infection of cells by canine distemper virus (CDV) was not impaired after cellular cholesterol had been depleted by the drug methyl-beta-cyclodextrin. This effect was independent of the multiplicity of infection and the cellular receptor used for infection. However, cholesterol depletion of the viral envelope significantly reduced CDV infectivity. Replenishment by addition of exogenous cholesterol restored infectivity up to 80%. Thus, we conclude that CDV entry is dependent on cholesterol in the viral envelope. Furthermore, reduced syncytium formation was observed when the cells were cholesterol depleted during the course of the infection. This may be related to the observation that CDV envelope proteins H and F partitioned into cellular detergent-resistant membranes. Therefore, a role for lipid rafts during virus assembly and release as well is suggested.
Figures
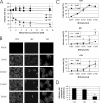

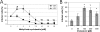


Similar articles
-
Hepatitis B virus infection is dependent on cholesterol in the viral envelope.Cell Microbiol. 2009 Feb;11(2):249-60. doi: 10.1111/j.1462-5822.2008.01250.x. Epub 2008 Nov 5. Cell Microbiol. 2009. PMID: 19016777
-
Mechanism of reduction of virus release and cell-cell fusion in persistent canine distemper virus infection.Acta Neuropathol. 2003 Oct;106(4):303-10. doi: 10.1007/s00401-003-0731-0. Epub 2003 Jun 21. Acta Neuropathol. 2003. PMID: 12827396
-
The fusion protein of wild-type canine distemper virus is a major determinant of persistent infection.Virology. 2005 Jul 5;337(2):312-26. doi: 10.1016/j.virol.2005.04.012. Virology. 2005. PMID: 15893783
-
Demyelination in canine distemper virus infection: a review.Acta Neuropathol. 2005 Jan;109(1):56-68. doi: 10.1007/s00401-004-0958-4. Epub 2005 Jan 12. Acta Neuropathol. 2005. PMID: 15645260 Review.
-
Pathogenesis and immunopathology of systemic and nervous canine distemper.Vet Immunol Immunopathol. 2009 Jan 15;127(1-2):1-18. doi: 10.1016/j.vetimm.2008.09.023. Epub 2008 Oct 4. Vet Immunol Immunopathol. 2009. PMID: 19019458 Review.
Cited by
-
Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2.Virology. 2008 Nov 25;381(2):215-21. doi: 10.1016/j.virol.2008.08.026. Epub 2008 Sep 23. Virology. 2008. PMID: 18814896 Free PMC article.
-
Overcoming the Barrier of the Respiratory Epithelium during Canine Distemper Virus Infection.mBio. 2022 Feb 22;13(1):e0304321. doi: 10.1128/mbio.03043-21. Epub 2022 Jan 18. mBio. 2022. PMID: 35038920 Free PMC article.
-
Cholesterol reducing agents inhibit assembly of type I parainfluenza viruses.Virology. 2017 Jan 15;501:127-135. doi: 10.1016/j.virol.2016.11.011. Epub 2016 Dec 2. Virology. 2017. PMID: 27915128 Free PMC article.
-
Safety and Immunogenicity of a Canine Distemper DNA Vaccine Formulated with Lipid Nanoparticles in Dogs, Foxes, and Raccoon Dogs.Vaccines (Basel). 2025 Jun 6;13(6):614. doi: 10.3390/vaccines13060614. Vaccines (Basel). 2025. PMID: 40573945 Free PMC article.
-
Critical role of cellular cholesterol in bovine rotavirus infection.Virol J. 2014 May 23;11:98. doi: 10.1186/1743-422X-11-98. Virol J. 2014. PMID: 24884772 Free PMC article.
References
-
- Ali, A., and D. P. Nayak. 2000. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology 276:289-303. - PubMed
-
- Anderton, P., T. F. Wild, and G. Zwingelstein. 1983. Accumulation of radiolabelled fatty acids in the neutral fraction of measles virus persistently infected BGM cells. Biochem. Biophys. Res. Commun. 112:29-34. - PubMed
-
- Bickel, P. E., P. E. Scherer, J. E. Schnitzer, P. Oh, M. P. Lisanti, and H. F. Lodish. 1997. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272:13793-13802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

