Postsynaptic membrane addition depends on the Discs-Large-interacting t-SNARE Gtaxin
- PMID: 17267557
- PMCID: PMC4664082
- DOI: 10.1523/JNEUROSCI.3160-06.2007
Postsynaptic membrane addition depends on the Discs-Large-interacting t-SNARE Gtaxin
Abstract
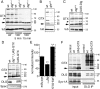
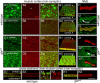
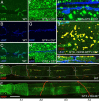
Targeted membrane addition is a hallmark of many cellular functions. In the nervous system, modification of synaptic membrane size has a major impact on synaptic function. However, because of the complex shape of neurons and the need to target membrane addition to very small and polarized synaptic compartments, this process is poorly understood. Here, we show that Gtaxin (GTX), a Drosophila t-SNARE (target-soluble N-ethylmaleimide-sensitive factor attachment protein receptor), is required for expansion of postsynaptic membranes during new synapse formation. Mutations in gtx lead to drastic reductions in postsynaptic membrane surface, whereas gtx upregulation results in the formation of complex membrane structures at ectopic sites. Postsynaptic GTX activity depends on its direct interaction with Discs-Large (DLG), a multidomain scaffolding protein of the PSD-95 (postsynaptic density protein-95) family with key roles in cell polarity and formation of cellular junctions as well as synaptic protein anchoring and trafficking. We show that DLG selectively determines the postsynaptic distribution of GTX to type I, but not to type II or type III boutons on the same cell, thereby defining sites of membrane addition to this unique set of glutamatergic synapses. We provide a mechanistic explanation for selective targeted membrane expansion at specific synaptic junctions.
Figures






References
-
- Cathala L, Holderith NB, Nusser Z, DiGregorio DA, Cull-Candy SG. Changes in synaptic structure underlie the developmental speeding of AMPA receptor-mediated EPSCs. Nat Neurosci. 2005;8:1310–1318. - PubMed
-
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000a;290:1364–1368. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
