Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1
- PMID: 17267560
- PMCID: PMC6673195
- DOI: 10.1523/JNEUROSCI.4583-06.2007
Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1
Abstract
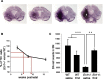
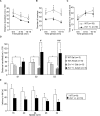
Engrailed1 and Engrailed2 (En1 and En2) are two developmental genes of the homeogene family expressed in the developing midbrain. En1 and, to a lesser degree, En2 also are expressed in the adult substantia nigra (SN) and ventral tegmental area (VTA), two dopaminergic (DA) nuclei of the ventral midbrain. In an effort to study En1/2 adult functions, we have analyzed the phenotype of mice lacking one En1 allele in an En2 wild-type context. We show that in this mutant the number of DA neurons decreases slowly between 8 and 24 weeks after birth to reach a stable 38 and 23% reduction in the SN and VTA, respectively, and that neuronal loss can be antagonized by En2 recombinant protein infusions in the midbrain. These loss and gain of function experiments firmly establish that En1/2 is a true survival factor for DA neurons in vivo. Neuronal death in the mutant is paralleled by a 37% decrease in striatal DA, with no change in serotonin content. Using established protocols, we show that, compared with their wild-type littermates, En1+/- mice have impaired motor skills, an anhedonic-like behavior, and an enhanced resignation phenotype; they perform poorly in social interactions. However, these mice do not differ from their wild-type littermates in anxiety-measuring tests. Together, these results demonstrate that En1/2 genes have important adult physiological functions. They also suggest that mice lacking only one En1 allele could provide a novel model for the study of diseases associated with progressive DA cell death.
Figures




References
-
- Alberi L, Sgado P, Simon HH. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131:3229–3236. - PubMed
-
- Borgkvist A, Puelles E, Carta M, Acampora D, Ang SL, Wurst W, Goiny M, Fisone G, Simeone A, Usiello A. Altered dopaminergic innervation and amphetamine response in adult Otx2 conditional mutant mice. Mol Cell Neurosci. 2006;31:293–302. - PubMed
-
- Casas M, Prat G, Robledo P, Barbanoj M, Kulisevsky J, Jane F. Methylxanthines reverse the adipsic and aphagic syndrome induced by bilateral 6-hydroxydopamine lesions of the nigrostriatal pathway in rats. Pharmacol Biochem Behav. 2000;66:257–263. - PubMed
-
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
