Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation
- PMID: 17267561
- PMCID: PMC6673189
- DOI: 10.1523/JNEUROSCI.4746-06.2007
Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation
Abstract
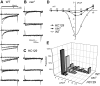
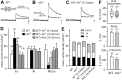
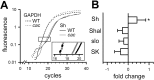
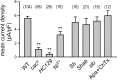
The cacophony (cac) locus in Drosophila encodes a Ca2+ channel alpha subunit, but little is known about properties of cac-mediated currents and functional consequences of cac mutations in central neurons. We found that, in Drosophila cultured neurons, Ca2+ currents were mediated predominantly by the cac channels. The cac channels contribute to low- and high-threshold, fast- and slow-inactivating types of Ca2+ currents, take part in membrane depolarization, and strongly activate Ca2+-activated K+ current [I(K(Ca))]. In cac neurons, unexpectedly, voltage-activated transient K+ current I(A) is upregulated to a level that matches I(K(Ca)) reduction, implicating a homeostatic regulation that was mimicked by chronic pharmacological blockade of Ca2+ currents in wild-type neurons. Among K+ channel transcripts, Shaker mRNA levels were preferentially increased in cac flies. However, Ca2+ current expression levels remained unaltered in several K+ channel mutants, illustrating a key role of cac in developmental regulation of Drosophila neuronal excitability.
Figures




Similar articles
-
Distinct roles of Drosophila cacophony and Dmca1D Ca(2+) channels in synaptic homeostasis: genetic interactions with slowpoke Ca(2+) -activated BK channels in presynaptic excitability and postsynaptic response.Dev Neurobiol. 2014 Jan;74(1):1-15. doi: 10.1002/dneu.22120. Epub 2013 Oct 7. Dev Neurobiol. 2014. PMID: 23959639 Free PMC article.
-
Cav2-type calcium channels encoded by cac regulate AP-independent neurotransmitter release at cholinergic synapses in adult Drosophila brain.J Neurophysiol. 2009 Jan;101(1):42-53. doi: 10.1152/jn.91103.2008. Epub 2008 Nov 12. J Neurophysiol. 2009. PMID: 19004991 Free PMC article.
-
Sub-cellular Ca2+ dynamics affected by voltage- and Ca2+-gated K+ channels: Regulation of the soma-growth cone disparity and the quiescent state in Drosophila neurons.Neuroscience. 2006 Oct 27;142(3):629-44. doi: 10.1016/j.neuroscience.2006.06.051. Epub 2006 Aug 17. Neuroscience. 2006. PMID: 16919393
-
Non-synaptic ion channels in insects--basic properties of currents and their modulation in neurons and skeletal muscles.Prog Neurobiol. 2001 Aug;64(5):431-525. doi: 10.1016/s0301-0082(00)00066-6. Prog Neurobiol. 2001. PMID: 11301158 Review.
-
Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents.Gen Pharmacol. 1998 Jan;30(1):13-24. doi: 10.1016/s0306-3623(97)00034-7. Gen Pharmacol. 1998. PMID: 9457476 Review.
Cited by
-
TRP, TRPL and cacophony channels mediate Ca2+ influx and exocytosis in photoreceptors axons in Drosophila.PLoS One. 2012;7(8):e44182. doi: 10.1371/journal.pone.0044182. Epub 2012 Aug 31. PLoS One. 2012. PMID: 22952921 Free PMC article.
-
Pumilio binds para mRNA and requires Nanos and Brat to regulate sodium current in Drosophila motoneurons.J Neurosci. 2008 Feb 27;28(9):2099-109. doi: 10.1523/JNEUROSCI.5092-07.2008. J Neurosci. 2008. PMID: 18305244 Free PMC article.
-
Modulator-Gated, SUMOylation-Mediated, Activity-Dependent Regulation of Ionic Current Densities Contributes to Short-Term Activity Homeostasis.J Neurosci. 2019 Jan 23;39(4):596-611. doi: 10.1523/JNEUROSCI.1379-18.2018. Epub 2018 Nov 30. J Neurosci. 2019. PMID: 30504282 Free PMC article.
-
Activation of high and low affinity dopamine receptors generates a closed loop that maintains a conductance ratio and its activity correlate.Front Neural Circuits. 2013 Oct 22;7:169. doi: 10.3389/fncir.2013.00169. eCollection 2013. Front Neural Circuits. 2013. PMID: 24155696 Free PMC article.
-
Ionic current correlations underlie the global tuning of large numbers of neuronal activity attributes.J Neurosci. 2012 Sep 26;32(39):13380-8. doi: 10.1523/JNEUROSCI.6500-11.2012. J Neurosci. 2012. PMID: 23015428 Free PMC article.
References
-
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Alkon DL. Ionic conductance determinants of synaptic memory nets and their implications for Alzheimer's disease. J Neurosci Res. 1999;58:24–32. - PubMed
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
-
- Baker K, Salkoff L. The Drosophila Shaker gene codes for a distinctive K+ current in a subset of neurons. Neuron. 1990;4:129–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
