Acute hypoxia activates the neuroimmune system, which diabetes exacerbates
- PMID: 17267571
- PMCID: PMC6673177
- DOI: 10.1523/JNEUROSCI.4560-06.2007
Acute hypoxia activates the neuroimmune system, which diabetes exacerbates
Abstract
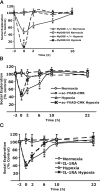
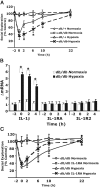
Acute hypoxia is experienced in an array of ailments and conditions, including asthma, chronic obstructive pulmonary disease, heart failure, sleep apnea, acute hypotension, and blast lung injury. Classically, infection activates the neuroimmune system, causing loss of interest in the social environment. We report that the non-infectious stimulus acute hypoxia triggers neuroimmune system activation (NSA), causing loss of interest in the social environment, and that recovery from hypoxia-induced NSA is impaired in a mouse model of type 2 diabetes. Importantly, recovery from the behavioral consequences of hypoxia-induced NSA was nearly ablated in MyD88 (myeloid differentiation factor 88) knock-out mice and in mice intracerebroventricularly administered the caspase-1 inhibitor ac-YVAD-CMK (ac-Tyr-Val-Asp-2,6-dimethylbenzoyloxymethylketone). Diabetic mice had prolonged recovery from NSA that could be halved by administration of subcutaneous interleukin-1 (IL-1) receptor antagonist (RA). These results show that acute hypoxia activates the IL-1beta arm of the neuroimmune system, which diabetes exacerbates and treatment with IL-1RA ameliorates.
Figures

Similar articles
-
Acute hypoxia, diabetes, and neuroimmune dysregulation: converging mechanisms in the brain.Neuroscientist. 2008 Jun;14(3):235-9. doi: 10.1177/1073858407309544. Epub 2007 Nov 13. Neuroscientist. 2008. PMID: 18000066 Review.
-
Behavioral recovery from acute hypoxia is reliant on leptin.Brain Behav Immun. 2009 Feb;23(2):169-75. doi: 10.1016/j.bbi.2008.09.011. Epub 2008 Sep 27. Brain Behav Immun. 2009. PMID: 18854211 Free PMC article.
-
[Caspase-1 inhibitor AC-YVAD-CMK blocks IL-1β secretion of bone marrow-derived macrophages induced by Acinetobacter baumannii].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017 Dec;33(12):1594-1599. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017. PMID: 29382416 Chinese.
-
Dual role of interleukin-1β in islet amyloid formation and its β-cell toxicity: Implications for type 2 diabetes and islet transplantation.Diabetes Obes Metab. 2017 May;19(5):682-694. doi: 10.1111/dom.12873. Epub 2017 Feb 27. Diabetes Obes Metab. 2017. PMID: 28058779
-
Targeting Imbalance between IL-1β and IL-1 Receptor Antagonist Ameliorates Delayed Epithelium Wound Healing in Diabetic Mouse Corneas.Am J Pathol. 2016 Jun;186(6):1466-80. doi: 10.1016/j.ajpath.2016.01.019. Epub 2016 Apr 22. Am J Pathol. 2016. PMID: 27109611 Free PMC article.
Cited by
-
Individually ventilated cages cause chronic low-grade hypoxia impacting mice hematologically and behaviorally.Brain Behav Immun. 2012 Aug;26(6):951-8. doi: 10.1016/j.bbi.2012.04.008. Epub 2012 May 3. Brain Behav Immun. 2012. PMID: 22561683 Free PMC article.
-
Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization.Brain Behav Immun. 2010 May;24(4):631-40. doi: 10.1016/j.bbi.2010.01.015. Epub 2010 Feb 6. Brain Behav Immun. 2010. PMID: 20138982 Free PMC article.
-
Hypoxia/reoxygenation impairs memory formation via adenosine-dependent activation of caspase 1.J Neurosci. 2012 Oct 3;32(40):13945-55. doi: 10.1523/JNEUROSCI.0704-12.2012. J Neurosci. 2012. PMID: 23035103 Free PMC article.
-
Handling stress impairs learning through a mechanism involving caspase-1 activation and adenosine signaling.Brain Behav Immun. 2019 Aug;80:763-776. doi: 10.1016/j.bbi.2019.05.025. Epub 2019 May 17. Brain Behav Immun. 2019. PMID: 31108171 Free PMC article.
-
Neuroimmune interaction in inflammatory diseases.Clin Med Circ Respirat Pulm Med. 2008 Apr 29;2:35-44. doi: 10.4137/ccrpm.s547. Clin Med Circ Respirat Pulm Med. 2008. PMID: 21157520 Free PMC article.
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. - PubMed
-
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–4456. - PubMed
-
- Bonnert TP, Garka KE, Parnet P, Sonoda G, Testa JR, Sims JE. The cloning and characterization of human MyD88: a member of an IL-1 receptor related family. FEBS Lett. 1997;402:81–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
