Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion
- PMID: 17267576
- PMCID: PMC6673179
- DOI: 10.1523/JNEUROSCI.4908-06.2007
Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion
Abstract
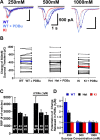
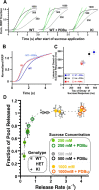
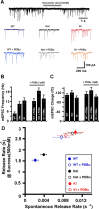
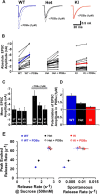
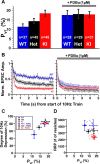
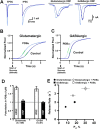
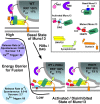
Synapses need to encode a wide dynamic range of action potential frequencies. Essential vesicle priming proteins of the Munc13 (mammalian Unc13) family play an important role in adapting vesicle supply to variable demand and thus influence short-term plasticity characteristics and synaptic function. Structure-function analyses of Munc13s have identified a "catalytic" C-terminal domain and several N-terminal modulatory domains, including a diacylglycerol/phorbol ester [4beta-phorbol-12, 13-dibutyrate (PDBu)] binding C1 domain. Although still allowing basal priming, a Munc13-1 C1 domain mutation (H567K) prevents PDBu induced potentiation of evoked transmitter release, leads to strong depression during trains of synaptic activity, and causes perinatal lethality in mice. To understand the mechanism of C1 domain-mediated modulation of Munc13 function, we examined how PDBu increases neurotransmitter release. Analyses of osmotically induced release as well as Ca2+ triggered and spontaneous release showed that PDBu increases the vesicular release rate without affecting the size of the readily releasable vesicle pool, linking C1 domain activation to a lowering of the energy barrier for vesicle fusion. PDBu binding-deficient mutant Munc13-1(H567K) synapses mirrored the vesicular release properties of PDBu-potentiated wild-type synapses, indicating that Munc13-1(H567K) is a gain-of-function mutant, which conformationally mimics the PDBu-activated state of Munc13-1. We propose a PKC analogous two-state model of regulation of Munc13s, in which the basal state of Munc13s is disinhibited by C1 domain activation into a state of facilitated vesicle release, regardless of whether the release is spontaneous or action potential triggered.
Figures
References
-
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J Biol Chem. 2006;281:19720–19731. - PubMed
-
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci. 1999;2:965–971. - PubMed
-
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
-
- Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, Grishin NV, Rosenmund C, Rizo J. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
