Signaling at A-kinase anchoring proteins organizes anesthesia-sensitive memory in Drosophila
- PMID: 17267579
- PMCID: PMC6673183
- DOI: 10.1523/JNEUROSCI.4622-06.2007
Signaling at A-kinase anchoring proteins organizes anesthesia-sensitive memory in Drosophila
Abstract
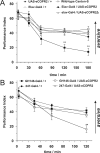
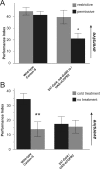
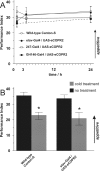
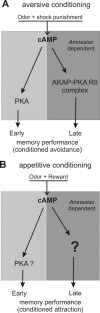
The ubiquitous cAMP-protein kinase A (PKA) signaling pathway exhibits complex temporal requirements during the time course of associative memory processing. This directly raises questions about the molecular mechanisms that provide signaling specificity to this pathway. Here, we use Drosophila olfactory conditioning to show that divergent cAMP signaling is mediated by functionally distinct pools of PKA. One particular pool is organized via the PKA regulatory type II subunit at the level of A-kinase anchoring proteins (AKAPs), a family of scaffolding proteins that provides focal points of spatiotemporal signal integration. This AKAP-bound pool of PKA is acting within neurons of the mushroom bodies to support a late phase of aversive memory. The requirement for AKAP-bound PKA signaling is limited to aversive memory, but dispensable during appetitive memory. This finding suggests the existence of additional mechanisms to support divergence within the cAMP-PKA signaling pathway during memory processing. Together, our results show that subcellular organization of signaling components plays a key role in memory processing.
Figures
References
-
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. - PubMed
-
- Banky P, Huang LJ, Taylor SS. Dimerization/docking domain of the type Ialpha regulatory subunit of cAMP-dependent protein kinase.Requirements for dimerization and docking are distinct but overlapping. J Biol Chem. 1998;273:35048–35055. - PubMed
-
- Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol. 1999;9:216–221. - PubMed
-
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. - PubMed
-
- DeZazzo J, Tully T. Dissection of memory formation: from behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
