Evaluation of the Partec flow cytometer against the BD FACSCalibur system for monitoring immune responses of human immunodeficiency virus-infected patients in Zimbabwe
- PMID: 17267593
- PMCID: PMC1828850
- DOI: 10.1128/CVI.00416-06
Evaluation of the Partec flow cytometer against the BD FACSCalibur system for monitoring immune responses of human immunodeficiency virus-infected patients in Zimbabwe
Abstract
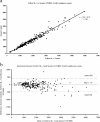
A single-platform volumetric flow cytometer, the Partec Cyflow SL_3, was evaluated against a BD FACSCalibur/Sysmex XT1800i dual platform for measuring CD4(+) lymphocytes, total lymphocytes, and the percentage of CD4 lymphocytes in whole-blood samples for monitoring the immune systems of human immunodeficiency virus (HIV)/AIDS patients. Statistical analyses for precision, correlation, and agreement were performed. Coefficients of variation (CV) of 5.8, 4.6, and 3.9% were obtained for low, medium, and high CD4(+) cell counts, respectively, using the SL_3, and CV of 3.7, 4.0, and 0.94 were obtained for the same categories, using the BD FACSCalibur. Significant correlations (P < 0.005) between the two assays for CD4 counts, total lymphocyte counts, and percentages of CD4 were obtained, with correlation coefficients of 0.99, 0.96, and 0.99, respectively (n = 229). Using the Bland-Altman plot, mean biases of -18 cell/microl (95% confidence interval (CI); -91 to 54 cells/microl), -0.8% (95% CI; -3.6 to 2%), and -36.8 cells/microl (95% CI; -477 to 404 cells/microl) were obtained for comparisons of CD4 counts, percentages of CD4 cells, and total lymphocyte counts, respectively. The effects of the age of the samples on the three parameters were also analyzed by comparing results from the same samples analyzed at 6, 24, and 48 h after collection. The correlation coefficients for comparisons among different time points for the same machine and among all the time points for the two different machines were greater than 0.90. These data showed that the Partec Cyflow SL_3 assay is comparable to the BD FACSCalibur/Sysmex XT1800i dual-platform method for measuring the amount of CD4(+) cells and total lymphocytes and the percentages of CD4 cells in blood samples for the purpose of monitoring HIV/AIDS patients.
Figures




Similar articles
-
Evaluation of a new single-parameter volumetric flow cytometer (CyFlow(green)) for enumeration of absolute CD4+ T lymphocytes in human immunodeficiency virus type 1-infected Thai patients.Clin Diagn Lab Immunol. 2005 Dec;12(12):1416-24. doi: 10.1128/CDLI.12.12.1416-1424.2005. Clin Diagn Lab Immunol. 2005. PMID: 16339065 Free PMC article.
-
Influence of 4- and 6-color flow cytometers and acquisition/analysis softwares on the determination of lymphocyte subsets in HIV infection.Cytometry B Clin Cytom. 2007 Sep;72(5):380-6. doi: 10.1002/cyto.b.20178. Cytometry B Clin Cytom. 2007. PMID: 17226862
-
Evaluation of a single-platform microcapillary flow cytometer for enumeration of absolute CD4+ T-lymphocyte counts in HIV-1 infected Thai patients.Cytometry B Clin Cytom. 2007 Sep;72(5):387-96. doi: 10.1002/cyto.b.20167. Cytometry B Clin Cytom. 2007. PMID: 17474130
-
Evaluation of a flow cytometry method for CD4 T cell enumeration based on volumetric primary CD4 gating using thermoresistant reagents.J Immunol Methods. 2011 Sep 30;372(1-2):7-13. doi: 10.1016/j.jim.2011.07.012. Epub 2011 Jul 29. J Immunol Methods. 2011. PMID: 21835181 Review.
-
Absolute and percent CD4+ T-cell enumeration by flow cytometry using capillary blood.J Immunol Methods. 2011 Sep 30;372(1-2):1-6. doi: 10.1016/j.jim.2011.07.008. Epub 2011 Jul 20. J Immunol Methods. 2011. PMID: 21787779 Review.
Cited by
-
Laboratory and field evaluation of the Partec CyFlow miniPOC for absolute and relative CD4 T-cell enumeration.PLoS One. 2015 Feb 17;10(2):e0116663. doi: 10.1371/journal.pone.0116663. eCollection 2015. PLoS One. 2015. PMID: 25688553 Free PMC article.
-
Diagnostic point-of-care tests in resource-limited settings.Lancet Infect Dis. 2014 Mar;14(3):239-49. doi: 10.1016/S1473-3099(13)70250-0. Epub 2013 Dec 10. Lancet Infect Dis. 2014. PMID: 24332389 Free PMC article. Review.
-
Low-cost assays for monitoring HIV infected individuals in resource-limited settings.Indian J Med Res. 2011 Dec;134(6):823-34. doi: 10.4103/0971-5916.92628. Indian J Med Res. 2011. PMID: 22310816 Free PMC article. Review.
-
Evaluation of PIMA™® point of care technology for CD4 T cell enumeration in Kenya.PLoS One. 2013 Jun 25;8(6):e67612. doi: 10.1371/journal.pone.0067612. Print 2013. PLoS One. 2013. PMID: 23825674 Free PMC article.
-
Single-platform, volumetric, CD45-assisted pan-leucogating flow cytometry for CD4 T lymphocytes monitoring of HIV infection according to the WHO recommendations for resource-constrained settings.BMC Res Notes. 2013 Apr 30;6:169. doi: 10.1186/1756-0500-6-169. BMC Res Notes. 2013. PMID: 23631664 Free PMC article.
References
-
- Bland, J., and D. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. - PubMed
-
- Carrieri, F., B. Spire, S. Duran, C. Katlama, D. Peyramond, C. Francois, G. Chene, J. Lang, J. Moatti, C. Leport, and the APROCO study group. 2003. Health related quality of life after 1 year of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 32:38-47. - PubMed
-
- Cassens, U., W. Gohde, G. Kuling, A. Groning, P. Schlenke, L. Lehman, Y. Taore, J. Servais, Y. Hemin, D. Reichelt, and B. Greve. 2004. Simple volumetric flow cytometry allows feasible and accurate determination of CD4 T-lymphocytes in immunodeficient patients worldwide. Antivir. Ther. 9:395-405. - PubMed
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 2006. Guidelines for the use of the antiretroviral agents in HIV-1 infected adults and adolescents. Office of AIDS Research Advisory Council, Bethesda, MD. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
-
- Federal Register. 1996. Notice of specific list for categorization of laboratory test systems, assays, and examinations by complexity; notice of additional waived laboratory test systems, assays, and examinations; and notice of announcement of boards approved by HHS. Fed. Regist. 61:35736-35762. http://www.fda.gov/cdrh/CLIA/fr/61fr35736.html.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

