Synthetic cooperation in engineered yeast populations
- PMID: 17267602
- PMCID: PMC1794266
- DOI: 10.1073/pnas.0610575104
Synthetic cooperation in engineered yeast populations
Abstract
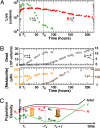
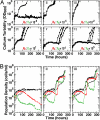
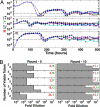
Cooperative interactions are key to diverse biological phenomena ranging from multicellularity to mutualism. Such diversity makes the ability to create and control cooperation desirable for potential applications in areas as varied as agriculture, pollutant treatment, and medicine. Here we show that persistent cooperation can be engineered by introducing a small set of genetic modifications into previously noninteracting cell populations. Specifically, we report the construction of a synthetic obligatory cooperative system, termed CoSMO (cooperation that is synthetic and mutually obligatory), which consists of a pair of nonmating yeast strains, each supplying an essential metabolite to the other strain. The behavior of the two strains in isolation, however, revealed unintended constraints that restrict cooperation, such as asymmetry in starvation tolerance and delays in nutrient release until near cell death. However, the joint system is shown mathematically and experimentally to be viable over a wide range of initial conditions, with oscillating population ratio settling to a value predicted by nutrient supply and consumption. Unexpectedly, even in the absence of explicitly engineered mechanisms to stabilize cooperation, the cooperative system can consistently develop increased ability to survive reductions in population density. Extending synthetic biology from the design of genetic circuits to the engineering of ecological interactions, CoSMO provides a quantitative system for linking processes at the cellular level to the collective behavior at the system level, as well as a genetically tractable system for studying the evolution of cooperation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Synthetic ecology: a model system for cooperation.Proc Natl Acad Sci U S A. 2007 Feb 6;104(6):1741-2. doi: 10.1073/pnas.0611067104. Epub 2007 Jan 31. Proc Natl Acad Sci U S A. 2007. PMID: 17267618 Free PMC article. No abstract available.
References
-
- Hanahan D, Folkman J. Cell. 1996;86:353–364. - PubMed
-
- Boucher DH. The Biology of Mutualism: Ecology and Evolution. New York: Oxford Univ Press; 1985.
-
- Bergstrom CT, Bronstein JL, Bshary R, Connor RC, Daly M, Frank SA, Gintis H, Keller L, Leimar O, Noe R, Queller DC. In: Genetic and Cultural Evolution of Cooperation. Hammerstein P, editor. Boston: MIT Press; 2002.
-
- Rainey PB, Rainey K. Nature. 2003;425:72–74. - PubMed
-
- Buchsbaum R, Buchsbaum M. Science. 1934;80:408–409. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

