Superoxide-mediated amplification of the oxygen-induced switch from [4Fe-4S] to [2Fe-2S] clusters in the transcriptional regulator FNR
- PMID: 17267605
- PMCID: PMC1892919
- DOI: 10.1073/pnas.0609514104
Superoxide-mediated amplification of the oxygen-induced switch from [4Fe-4S] to [2Fe-2S] clusters in the transcriptional regulator FNR
Abstract
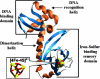
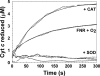
In Escherichia coli, the switch between aerobic and anaerobic metabolism is controlled primarily by FNR (regulator of fumarate and nitrate reduction), the protein that regulates the transcription of >100 genes in response to oxygen. Under oxygen-limiting conditions, FNR binds a [4Fe-4S]2+ cluster, generating a transcriptionally active dimeric form. Upon exposure to oxygen the cluster converts to a [2Fe-2S]2+ form, leading to dissociation of the protein into monomers, which are incapable of binding DNA with high affinity. The mechanism of cluster conversion together with the nature of the products of conversion is of considerable current interest. Here, we demonstrate that [4Fe-4S]2+ to [2Fe-2S]2+ cluster conversion, in both native and reconstituted [4Fe-4S] FNR, proceeds via a one electron oxidation of the cluster, to give a [3Fe-4S]1+ cluster intermediate, with the release of one Fe2+ ion and a superoxide ion. The cluster intermediate subsequently rearranges spontaneously to form the [2Fe-2S]2+ cluster, with the release of a Fe3+ ion and, as previously shown, two sulfide ions. Superoxide ion undergoes dismutation to hydrogen peroxide and oxygen. This mechanism, a one electron activation of the cluster, coupled to catalytic recycling of the resulting superoxide ion back to oxygen, provides a means of amplifying the sensitivity of [4Fe-4S] FNR to its signal molecule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
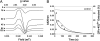


References
-
- Green J, Paget MS. Nat Rev Microbiol. 2004;2:954–966. - PubMed
-
- Guest JR. Philos Trans R Soc London B. 1995;350:189–202. - PubMed
-
- Guest JR. J Gen Microbiol. 1992;138:2253–2263. - PubMed
-
- Green J, Scott C, Guest JR. Adv Microb Physiol. 2001;44:1–34. - PubMed
-
- Korner H, Sofia HJ, Zumft WG. FEMS Microbiol Rev. 2003;27:559–592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

