Lack of myostatin results in excessive muscle growth but impaired force generation
- PMID: 17267614
- PMCID: PMC1794294
- DOI: 10.1073/pnas.0604893104
Lack of myostatin results in excessive muscle growth but impaired force generation
Erratum in
- Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):4240
Abstract
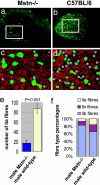
The lack of myostatin promotes growth of skeletal muscle, and blockade of its activity has been proposed as a treatment for various muscle-wasting disorders. Here, we have examined two independent mouse lines that harbor mutations in the myostatin gene, constitutive null (Mstn(-/-)) and compact (Berlin High Line, BEH(c/c)). We report that, despite a larger muscle mass relative to age-matched wild types, there was no increase in maximum tetanic force generation, but that when expressed as a function of muscle size (specific force), muscles of myostatin-deficient mice were weaker than wild-type muscles. In addition, Mstn(-/-) muscle contracted and relaxed faster during a single twitch and had a marked increase in the number of type IIb fibers relative to wild-type controls. This change was also accompanied by a significant increase in type IIB fibers containing tubular aggregates. Moreover, the ratio of mitochondrial DNA to nuclear DNA and mitochondria number were decreased in myostatin-deficient muscle, suggesting a mitochondrial depletion. Overall, our results suggest that lack of myostatin compromises force production in association with loss of oxidative characteristics of skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- McPherron AC, Lawler AM, Lee SJ. Nature. 1997;387:83–90. - PubMed
-
- Lee SJ. Annu Rev Cell Dev Biol. 2004;20:61–86. - PubMed
-
- Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. N Engl J Med. 2004;350:2682–2688. - PubMed
-
- Ferrell RE, Conte V, Lawrence EC, Roth SM, Hagberg JM, Hurley BF. Genomics. 1999;62:203–207. - PubMed
-
- Wagner KR, McPherron AC, Winik N, Lee SJ. Ann Neurol. 2002;52:832–836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

