Evaluation of the performance of the automated NucliSENS easyMAG and EasyQ systems versus the Roche AmpliPrep-AMPLICOR combination for high-throughput monitoring of human immunodeficiency virus load
- PMID: 17267632
- PMCID: PMC1865857
- DOI: 10.1128/JCM.01540-06
Evaluation of the performance of the automated NucliSENS easyMAG and EasyQ systems versus the Roche AmpliPrep-AMPLICOR combination for high-throughput monitoring of human immunodeficiency virus load
Abstract
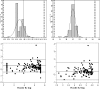
This study presents the data of an evaluation of the automated Nuclisens easyMAG and EasyQ systems versus the Roche AmpliPrep-AMPLICOR combination for testing of high-volume human immunodeficiency virus (HIV) load. This represents a follow-up of a previous study investigating the performance of the real-time Nuclisens assay using the semiautomated NucliSENS miniMAG extraction procedure. Three hundred eighteen patient samples were analyzed using both methods. The easyMAG-EasyQ HIV type 1 system has a higher sensitivity and broader dynamic range than the Cobas AmpliPrep-AMPLICOR system when the standard Roche assay is used alone, 25 to 3,000,000 IU/ml versus 400 to 750,000 HIV RNA copies/ml, respectively. There was significant correlation between the assays (0.93; P < 0.0001), with good accuracy (percent similarity mean mu = 96%), good precision (percent similarity standard deviation = 4.97%), and overall good agreement with a low percent similarity coefficient of variation of 5.17 to 6.11%. Bland-Altman analysis revealed that the AMPLICOR assay generated higher values than the EasyQ combination, with 95% of results within clinically acceptable limits. The throughput of samples was greatly improved using the easyMAG-EasyQ system, allowing 144 samples to be processed within 6 h. The potential for contamination has been dramatically reduced using the automated extraction system. Additional negative controls have been added to the kit to monitor for contamination based on the South African experience. This assay thus presents a real option for monitoring HIV load assays in high-volume testing environments.
Figures


References
-
- Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. - PubMed
-
- de Mendoza, C., M. Koppelman, B. Montes, V. Ferre, V. Soriano, H. Cuypers, M. Segondy, and T. Oosterlaken. 2005. Multicenter evaluation of the NucliSens EasyQ HIV-1 v1.1 assay for the quantitative detection of HIV-1 RNA in plasma. J. Virol. Methods 127:54-59. - PubMed
-
- National Department of Health. 2004. National antiretroviral treatment guidelines. National Department of Health, Johannesburg, South Africa. http://www.doh.gov.za/docs/factsheets/guidelines/artguide04-f.html.
-
- Scott, L. E., J. S. Galpin, and D. K. Glencross. 2003. Multiple method comparison: statistical model using percentage similarity. Cytometry B Clin. Cytom. 54:46-53. - PubMed
-
- Stevens, W., T. Wiggill, P. Horsfield, L. Coetzee, and L. E. Scott. 2005. Evaluation of the NucliSens EasyQ assay in HIV-1-infected individuals in South Africa. J. Virol. Methods 124:105-110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

