Subnuclear localization and dynamics of the Pre-mRNA 3' end processing factor mammalian cleavage factor I 68-kDa subunit
- PMID: 17267687
- PMCID: PMC1838998
- DOI: 10.1091/mbc.e06-09-0846
Subnuclear localization and dynamics of the Pre-mRNA 3' end processing factor mammalian cleavage factor I 68-kDa subunit
Abstract
Mammalian cleavage factor I (CF Im) is an essential factor that is required for the first step in pre-mRNA 3' end processing. Here, we characterize CF Im68 subnuclear distribution and mobility. Fluorescence microscopy reveals that in addition to paraspeckles CF Im68 accumulates in structures that partially overlap with nuclear speckles. Analysis of synchronized cells shows that CF Im68 distribution in speckles and paraspeckles varies during the cell cycle. At an ultrastructural level, CF Im68 is associated with perichromatin fibrils, the sites of active transcription, and concentrates in interchromatin granules-associated zones. We show that CFIm68 colocalizes with bromouridine, RNA polymerase II, and the splicing factor SC35. On inhibition of transcription, endogenous CF Im68 no longer associates with perichromatin fibrils, but it can still be detected in interchromatin granules-associated zones. These observations support the idea that not only splicing but also 3' end processing occurs cotranscriptionally. Finally, fluorescence recovery after photobleaching analysis reveals that the CF Im68 fraction associated with paraspeckles moves at a rate similar to the more dispersed molecules in the nucleoplasm, demonstrating the dynamic nature of this compartment. These findings suggest that paraspeckles are a functional compartment involved in RNA metabolism in the cell nucleus.
Figures
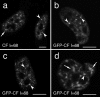

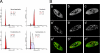
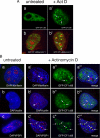


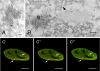


References
-
- Andersen J. S., Lyon C. E., Fox A. H., Leung A. K., Lam Y. W., Steen H., Mann M., Lamond A. I. Directed proteomic analysis of the human nucleolus. Curr. Biol. 2002;12:1–11. - PubMed
-
- Bentley D. The mRNA assembly line: transcription and processing machines in the same factory. Curr. Opin. Cell Biol. 2002;14:336–342. - PubMed
-
- Bernhard W. A new staining procedure for electron microscopical cytology. J. Ultrastruct. Res. 1969;27:250–265. - PubMed
-
- Biggiogera M., Fakan S. Fine structural specific visualization of RNA on ultrathin sections. J. Histochem. Cytochem. 1998;46:389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

