Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging
- PMID: 17268028
- PMCID: PMC3263830
Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging
Abstract
Recent developments have established molecular imaging of mouse models with small-animal PET and bioluminescence imaging (BLI) as an important tool in cancer research. One of the disadvantages of these imaging modalities is the lack of anatomic information. We combined small-animal PET and BLI technology with small-animal CT to obtain fusion images with both molecular and anatomic information.
Methods: We used small-animal PET/CT and BLI to detect xenografts of different cell lines and metastases of a melanoma cell line (A375M-3F) that had been transduced with a lentiviral vector containing a trimodality imaging reporter gene encoding a fusion protein with Renilla luciferase, monomeric red fluorescent protein, and a mutant herpes simplex virus type 1 thymidine kinase.
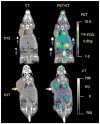
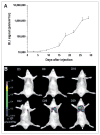
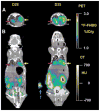
Results: Validation studies in mouse xenograft models showed a good coregistration of images from both PET and CT. Melanoma metastases were detected by 18F-FDG PET, 9-[4-(18)F-fluoro-3-(hydroxymethyl)butyl]guanine (18F-FHBG) PET, CT, and BLI and confirmed by ex vivo assays of Renilla luciferase and mutant thymidine kinase expression. 18F-FHBG PET/CT allowed detection and localization of lesions that were not seen on CT because of poor contrast resolution and were not seen on 18F-FDG PET because of higher background uptake relative to 18F-FHBG.
Conclusion: The combination of 18F-FHBG PET, small-animal CT, and BLI allows a sensitive and improved quantification of tumor burden in mice. This technique is potentially useful for the study of the biologic determinants of metastasis and for the evaluation of novel cancer treatments.
Figures




References
-
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
-
- Yu Y, Annala AJ, Barrio JR, et al. Quantification of target gene expression by imaging reporter gene expression in living animals. Nat Med. 2000;6:933–937. - PubMed
-
- Le LQ, Kabarowski JH, Wong S, Nguyen K, Gambhir SS, Witte ON. Positron emission tomography imaging analysis of G2A as a negative modifier of lymphoid leukemogenesis initiated by the BCR-ABL oncogene. Cancer Cell. 2002;1:381–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
