Efficient incorporation of a functional hyper-stable single-chain antibody fragment protein-IX fusion in the adenovirus capsid
- PMID: 17268536
- PMCID: PMC2233715
- DOI: 10.1038/sj.gt.3302908
Efficient incorporation of a functional hyper-stable single-chain antibody fragment protein-IX fusion in the adenovirus capsid
Abstract
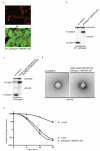
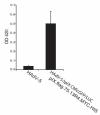
Recombinant adenoviruses are frequently used as gene transfer vehicles for therapeutic gene delivery. Strategies to amend their tropism include the incorporation of polypeptides with high affinity for cellular receptors. Single-chain antibodies have a great potential to achieve such cell type specificity. In this study, we evaluated the efficiency of incorporation of a single-chain antibody fused with the adenovirus minor capsid protein IX in the capsid of adenovirus type 5 vectors. To this end, the codons for the single-chain antibody fragments (scFv) 13R4 were fused with those encoding of pIX via a 75-Angstrom spacer sequence. The 13R4 is a hyper-stable single-chain antibody directed against beta-galactosidase, which was selected for its capacity to fold correctly in a reducing environment such as the cytoplasm. A lentiviral vector was used to stably express the pIX.flag.75.13R4.MYC.HIS fusion gene in 911 helper cells. Upon propagation of pIX-gene deleted human adenovirus-5 vectors on these cells, the pIX-fusion protein was efficiently incorporated in the capsid. Here, the 13R4 scFv was functional as was evident from its capacity to bind its ligand beta-galactosidase. These data demonstrate that the minor capsid protein IX can be used as an anchor for incorporation of single-chain antibodies in the capsids of adenovirus vectors.
Figures


Similar articles
-
Spacers increase the accessibility of peptide ligands linked to the carboxyl terminus of adenovirus minor capsid protein IX.J Virol. 2004 Apr;78(7):3470-9. doi: 10.1128/jvi.78.7.3470-3479.2004. J Virol. 2004. PMID: 15016870 Free PMC article.
-
Retargeting of adenovirus vectors through genetic fusion of a single-chain or single-domain antibody to capsid protein IX.J Virol. 2010 Oct;84(19):10074-86. doi: 10.1128/JVI.02665-09. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631131 Free PMC article.
-
A system for efficient generation of adenovirus protein IX-producing helper cell lines.J Gene Med. 2006 Feb;8(2):147-54. doi: 10.1002/jgm.844. J Gene Med. 2006. PMID: 16288495
-
Adenovirus protein IX: a new look at an old protein.Mol Ther. 2005 Jan;11(1):19-25. doi: 10.1016/j.ymthe.2004.09.018. Mol Ther. 2005. PMID: 15585402 Review.
-
Capsid Engineering of Adenovirus Vectors: Overcoming Early Vector-Host Interactions for Therapy.Hum Gene Ther. 2017 Oct;28(10):820-832. doi: 10.1089/hum.2017.139. Hum Gene Ther. 2017. PMID: 28854810 Review.
Cited by
-
Novel infectivity-enhanced oncolytic adenovirus with a capsid-incorporated dual-imaging moiety for monitoring virotherapy in ovarian cancer.Mol Imaging. 2009 Sep-Oct;8(5):264-77. Mol Imaging. 2009. PMID: 19796604 Free PMC article.
-
Protective immunity against a lethal respiratory Yersinia pestis challenge induced by V antigen or the F1 capsular antigen incorporated into adenovirus capsid.Hum Gene Ther. 2010 Jul;21(7):891-901. doi: 10.1089/hum.2009.148. Hum Gene Ther. 2010. PMID: 20180652 Free PMC article.
-
Targeting of adenovirus serotype 5 pseudotyped with short fiber from serotype 41 to c-erbB2-positive cells using bispecific single-chain diabody.J Mol Biol. 2009 May 8;388(3):443-61. doi: 10.1016/j.jmb.2009.03.016. Epub 2009 Mar 13. J Mol Biol. 2009. PMID: 19285990 Free PMC article.
-
The alphavirus nonstructural protein 2 NTPase induces a host translational shut-off through phosphorylation of eEF2 via cAMP-PKA-eEF2K signaling.PLoS Pathog. 2023 Feb 27;19(2):e1011179. doi: 10.1371/journal.ppat.1011179. eCollection 2023 Feb. PLoS Pathog. 2023. PMID: 36848386 Free PMC article.
-
Adenoviral targeting using genetically incorporated camelid single variable domains.Lab Invest. 2014 Aug;94(8):893-905. doi: 10.1038/labinvest.2014.82. Epub 2014 Jun 16. Lab Invest. 2014. PMID: 24933423 Free PMC article.
References
-
- St George JA. Gene therapy progress and prospects: adenoviral vectors. Gene Ther. 2003;10:1135–1141. - PubMed
-
- Magnusson MK, Hong SS, Henning P, Boulanger P, Lindholm L. Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. J Gene Med. 2002;4:356–370. - PubMed
-
- Biocca S, Ruberti F, Tafani M, Pierandrei-Amaldi P, Cattaneo A. Redox state of single chain Fv fragments targeted to the endoplasmic reticulum, cytosol and mitochondria. Biotechnology (N Y) 1995;13:1110–1115. - PubMed
-
- Hedley SJ, Auf der MA, Hohn S, Escher D, Barberis A, Glasgow JN, et al. An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther. 2006;13:88–94. - PubMed
-
- Cattaneo A, Biocca S. The selection of intracellular antibodies. Trends Biotechnol. 1999;17:115–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

