Structural basis of T-cell specificity and activation by the bacterial superantigen TSST-1
- PMID: 17268555
- PMCID: PMC1852840
- DOI: 10.1038/sj.emboj.7601531
Structural basis of T-cell specificity and activation by the bacterial superantigen TSST-1
Abstract
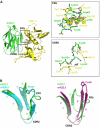
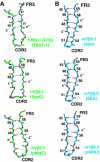
Superantigens (SAGs) bind simultaneously to major histocompatibility complex (MHC) and T-cell receptor (TCR) molecules, resulting in the massive release of inflammatory cytokines that can lead to toxic shock syndrome (TSS) and death. A major causative agent of TSS is toxic shock syndrome toxin-1 (TSST-1), which is unique relative to other bacterial SAGs owing to its structural divergence and its stringent TCR specificity. Here, we report the crystal structure of TSST-1 in complex with an affinity-matured variant of its wild-type TCR ligand, human T-cell receptor beta chain variable domain 2.1. From this structure and a model of the wild-type complex, we show that TSST-1 engages TCR ligands in a markedly different way than do other SAGs. We provide a structural basis for the high TCR specificity of TSST-1 and present a model of the TSST-1-dependent MHC-SAG-TCR T-cell signaling complex that is structurally and energetically unique relative to those formed by other SAGs. Our data also suggest that protein plasticity plays an exceptionally significant role in this affinity maturation process that results in more than a 3000-fold increase in affinity.
Figures



References
-
- Andersen PS, Lavoie PM, Sekaly RP, Churchill H, Kranz DM, Schlievert PM, Karjalainen K, Mariuzza RA (1999) Role of the T cell receptor alpha chain in stabilizing TCR–superantigen–MHC class II complexes. Immunity 10: 473–483 - PubMed
-
- Andersen PS, Schuck P, Sundberg EJ, Geisler C, Karjalainen K, Mariuzza RA (2002) Quantifying the energetics of cooperativity in a ternary protein complex. Biochemistry 41: 5177–5184 - PubMed
-
- Bentley GA, Boulot G, Karjalainen K, Mariuzza RA (1995) Crystal structure of the beta chain of a T cell antigen receptor. Science 267: 1984–1987 - PubMed
-
- Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP (1981) A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet 1: 1017–1021 - PubMed
-
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr, D 54: 905–921 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

