A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects
- PMID: 17268618
- PMCID: PMC4028372
- DOI: 10.1039/b612966h
A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects
Abstract
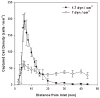
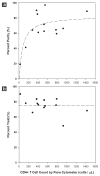
Practical HIV diagnostics are urgently needed in resource-limited settings. While HIV infection can be diagnosed using simple, rapid, lateral flow immunoassays, HIV disease staging and treatment monitoring require accurate counting of a particular white blood cell subset, the CD4(+) T lymphocyte. To address the limitations of current expensive, technically demanding and/or time-consuming approaches, we have developed a simple CD4 counting microfluidic device. This device uses cell affinity chromatography operated under differential shear flow to specifically isolate CD4(+) T lymphocytes with high efficiency directly from 10 microliters of unprocessed, unlabeled whole blood. CD4 counts are obtained under an optical microscope in a rapid, simple and label-free fashion. CD4 counts determined in our device matched measurements by conventional flow cytometry among HIV-positive subjects over a wide range of absolute CD4 counts (R(2) = 0.93). This CD4 counting microdevice can be used for simple, rapid and affordable CD4 counting in point-of-care and resource-limited settings.
Figures




References
-
- Joint United Nations Programme on HIV/AIDS. World Health Organization; Dec, 2005. [accessed 20th March 2006]. AIDS Epidemic Update: December 2005. available at http://libdoc.who.int/unaids/2005/929173439X_eng.pdf.
-
- Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; Oct 6, 2005. [accessed 20th March 2006]. available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
-
- Obrien WA, Hartigan PM, Daar ES, Simberkoff MS, Hamilton JD. Ann Intern Med. 1997;126:939–945. - PubMed
-
- Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR. Ann Intern Med. 1997;126:946–954. - PubMed
-
- Obrien WA, Hartigan PM, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Lahart C, Wray N, Finegold SM, George WL, Dickinson GM, Klimas N, Diamond G, ZollaPazner SB, Jensen PC, Hawkes C, Oster C, Gordin F, Labriola AM, Spivey P, Matthews T, Weinhold K, Drusano G, Egorin MJ. N Engl J Med. 1996;334:426–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

