Associative pairing enhances action potential back-propagation in radial oblique branches of CA1 pyramidal neurons
- PMID: 17272353
- PMCID: PMC2075451
- DOI: 10.1113/jphysiol.2006.121343
Associative pairing enhances action potential back-propagation in radial oblique branches of CA1 pyramidal neurons
Abstract
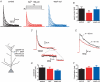
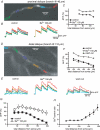
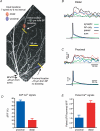
Back-propagating action potentials (bAPs) are involved in associative synaptic plasticity and the modulation of dendritic excitability. We have used high-speed confocal and two-photon imaging to measure calcium and voltage signals associated with action potential propagation into oblique branches of CA1 pyramidal neurons in adult hippocampal slices. The spatial profile of the bAP-associated Ca(2+) influx was biphasic, with an initial increase in the proximity of the branch point followed by a progressive decrease. Voltage imaging in the branches showed that bAP amplitude was initially constant and then steadily declined with distance from the soma. To determine the role of transient K(+) channels in this profile, we used external Ba(2+) (150 microm) as a channel blocker, after characterizing its effect on A-type K(+) channels in the apical trunk. Bath application of Ba(2+) significantly reduced the A-type K(+) current in outside-out patches and nearly eliminated the distance-dependent decrease in bAP amplitude and its associated Ca(2+) signal. Finally, small amplitude bAPs at more distal oblique branch locations could be boosted by simultaneous branch depolarization, such that the paired Ca(2+) signal became nearly the same for proximal and distal oblique dendrites. These data suggest that dendritic K(+) channels regulate the amplitude of bAPs to create a dendritic Ca(2+) signal whose magnitude is inversely related to the electrotonic distance from the soma when bAPs are not associated with a significant amount of localized synaptic input. This distance-dependent Ca(2+) signal from bAPs, however, can be amplified and a strong associative signal is produced once the proper correlation between synaptic activation and AP output is achieved. We hypothesize that these two signals may be involved in the regulation of the expression and activity of dendritic voltage- and ligand-gated ion channels.
Figures






References
-
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:150–170. - PubMed
-
- Antic S, Major G, Zecevic D. Fast optical recordings of membrane potential changes from dendrites of pyramidal neurons. J Neurophysiol. 1999;82:1615–1621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

