Synthetic 3-O-methylmannose-containing polysaccharides (sMMPs): design and synthesis
- PMID: 17274657
- PMCID: PMC2526461
- DOI: 10.1021/jo061991n
Synthetic 3-O-methylmannose-containing polysaccharides (sMMPs): design and synthesis
Erratum in
- J Org Chem. 2007 May 11;72(10):3980
Abstract
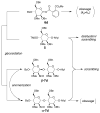
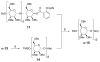
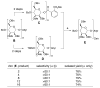
With the hope of mimicking the chemical and biological properties of natural 3-O-methylmannose-containing polysaccharides (MMPs), synthetic 3-O-methylmannose-containing polysaccharides (sMMPs) were designed and synthesized in a convergent manner. With little modification of the Mukaiyama glycosidation, high alpha-selectivity (>50:1 approximately >20:1) and yields (79 approximately 74%) were achieved for the key glycosidation steps. The exceptionally high alpha-selectivity observed was shown to be consequent to the selective anomerization of beta- to alpha-anomer under the glycosidation conditions. This glycosidation is well suited for a highly convergent oligosaccharide synthesis, particularly because of excellent chemical yields even when using approximately equal-sized donors and acceptors in an approximately 1:1 molar ratio. An iterative reaction sequence allowed the growing oligosaccharide to double in size after each cycle and led to an efficient synthesis of sMMP 8-, 12-, and 16-mers 18-20.
Figures

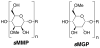
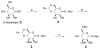
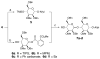

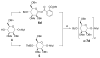
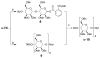
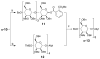
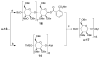

Similar articles
-
Highly stereoselective and iterative synthesis of alpha-(1-->4)-linked polysaccharides composed of 3-O-methyl-D-mannose.Org Lett. 2007 Aug 16;9(17):3323-6. doi: 10.1021/ol0713335. Epub 2007 Jul 21. Org Lett. 2007. PMID: 17658839
-
Synthetic 6-O-methylglucose-containing polysaccharides (sMGPs): design and synthesis.J Org Chem. 2007 Mar 16;72(6):1941-50. doi: 10.1021/jo061990v. Epub 2007 Feb 3. J Org Chem. 2007. PMID: 17274656 Free PMC article.
-
The α-Glycosidation of Partially Unprotected N-Acetyl and N-Glycolyl Sialyl Donors in the Absence of a Nitrile Solvent Effect.Chemistry. 2016 May 10;22(20):6968-73. doi: 10.1002/chem.201601031. Epub 2016 Apr 8. Chemistry. 2016. PMID: 27060996
-
Synthesis of D-galactofuranose-containing molecules: design of galactofuranosyl acceptors.Chembiochem. 2014 Jan 24;15(2):188-204. doi: 10.1002/cbic.201300638. Epub 2014 Jan 13. Chembiochem. 2014. PMID: 24420700 Review.
-
Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity.Acc Chem Res. 2018 Mar 20;51(3):628-639. doi: 10.1021/acs.accounts.7b00449. Epub 2018 Feb 22. Acc Chem Res. 2018. PMID: 29469568 Review.
Cited by
-
Protecting group-free glycoligation by the desulfurative rearrangement of allylic disulfides as a means of assembly of oligosaccharide mimetics.J Org Chem. 2011 May 20;76(10):3691-709. doi: 10.1021/jo102411j. Epub 2011 Mar 23. J Org Chem. 2011. PMID: 21428425 Free PMC article.
-
Synthesis of a Diacetonide-Protected, Mannose-Based Oxepine: Configurational Control of Anomeric Acetate Activation.J Org Chem. 2022 Jun 3;87(11):7474-7479. doi: 10.1021/acs.joc.2c00206. Epub 2022 May 16. J Org Chem. 2022. PMID: 35576505 Free PMC article.
-
Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions.Science. 2017 Jan 13;355(6321):162-166. doi: 10.1126/science.aal1875. Science. 2017. PMID: 28082586 Free PMC article.
References
-
-
For reviews on MMP and MGLP/MGP, see: Bloch K. Advances in Enzymology. 1977;45:1.Ballou CE. Accts Chem Res. 1968;1:366.Ballou CE. Pure Appl Chem. 1981;53:107.
-
-
-
For isolation and structural characterization of MMP, see: Gray GR, Ballou CE. J Biol Chem. 1971;246:6835.Maitra SK, Ballou CE. J Biol Chem. 1977;252:2459.Weisman LS, Ballou CE. J Biol Chem. 1984;259:3457.Weisman LS, Ballou CE. J Biol Chem. 1984;259:3464.Ilton M, Jevans AW, McCarthy ED, Vance D, White HB, III, Bloch K. Proc Natl Acad Sci USA. 1971;68:87.
-
-
-
For isolation and structural characterization of MGLP/MGP, see: Lee YC, Ballou CE. J Biol Chem. 1964;239:PC3602.Saier MH, Jr, Ballou CE. J Biol Chem. 1968;243:4332.Smith WL, Ballou CE. J Biol Chem. 1973;248:7118.Forsberg LS, Dell A, Walton DJ, Ballou CE. J Biol Chem. 1982;257:3555. Regarding the structural heterogeneity of MGP, Ballou commented that at least two forms of MGP containing 21 hexoses exist: Kamisango K, Dell A, Ballou CE. J Biol Chem. 1987;262:4580. Also see, Tuffal G, Albigot R, Monsarrat B, Ponthus C, Picard C, Rivière M, Puzo G. J Carbohydr Chem. 1995;14:631.
-
-
-
The structure of MG(L)P shown in Figure 1 is the revised structure suggested by Rivière based on the structure of polysaccharide isolated from Mycobacterium bovis BCG. See: Tuffal G, Albigot R, Rivière M, Puzo G. Glycobiology. 1998;8:675–684.
-
-
- Flick PK, Bloch K. J Biol Chem. 1974;249:1031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

