Environmental dimensionality controls the interaction of phagocytes with the pathogenic fungi Aspergillus fumigatus and Candida albicans
- PMID: 17274685
- PMCID: PMC1790725
- DOI: 10.1371/journal.ppat.0030013
Environmental dimensionality controls the interaction of phagocytes with the pathogenic fungi Aspergillus fumigatus and Candida albicans
Abstract
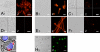
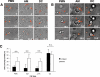
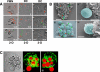
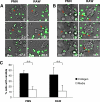
The fungal pathogens Aspergillus fumigatus and Candida albicans are major health threats for immune-compromised patients. Normally, macrophages and neutrophil granulocytes phagocytose inhaled Aspergillus conidia in the two-dimensional (2-D) environment of the alveolar lumen or Candida growing in tissue microabscesses, which are composed of a three-dimensional (3-D) extracellular matrix. However, neither the cellular dynamics, the per-cell efficiency, the outcome of this interaction, nor the environmental impact on this process are known. Live imaging shows that the interaction of phagocytes with Aspergillus or Candida in 2-D liquid cultures or 3-D collagen environments is a dynamic process that includes phagocytosis, dragging, or the mere touching of fungal elements. Neutrophils and alveolar macrophages efficiently phagocytosed or dragged Aspergillus conidia in 2-D, while in 3-D their function was severely impaired. The reverse was found for phagocytosis of Candida. The phagocytosis rate was very low in 2-D, while in 3-D most neutrophils internalized multiple yeasts. In competitive assays, neutrophils primarily incorporated Aspergillus conidia in 2-D and Candida yeasts in 3-D despite frequent touching of the other pathogen. Thus, phagocytes show activity best in the environment where a pathogen is naturally encountered. This could explain why "delocalized" Aspergillus infections such as hematogeneous spread are almost uncontrollable diseases, even in immunocompetent individuals.
Conflict of interest statement
Figures


References
-
- Brakhage AA. Systemic fungal infections caused by Aspergillus species: Epidemiology, infection process and virulence determinants. Curr Drug Targets. 2005;6:875–886. - PubMed
-
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. - PubMed
-
- Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. - PubMed
-
- Sole A, Morant P, Salavert M, Peman J, Morales P. Aspergillus infections in lung transplant recipients: Risk factors and outcome. Clin Microbiol Infect. 2005;11:359–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

