Antifungal chemical compounds identified using a C. elegans pathogenicity assay
- PMID: 17274686
- PMCID: PMC1790726
- DOI: 10.1371/journal.ppat.0030018
Antifungal chemical compounds identified using a C. elegans pathogenicity assay
Abstract
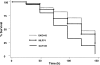
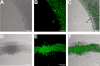
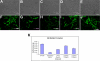
There is an urgent need for the development of new antifungal agents. A facile in vivo model that evaluates libraries of chemical compounds could solve some of the main obstacles in current antifungal discovery. We show that Candida albicans, as well as other Candida species, are ingested by Caenorhabditis elegans and establish a persistent lethal infection in the C. elegans intestinal track. Importantly, key components of Candida pathogenesis in mammals, such as filament formation, are also involved in nematode killing. We devised a Candida-mediated C. elegans assay that allows high-throughput in vivo screening of chemical libraries for antifungal activities, while synchronously screening against toxic compounds. The assay is performed in liquid media using standard 96-well plate technology and allows the study of C. albicans in non-planktonic form. A screen of 1,266 compounds with known pharmaceutical activities identified 15 (approximately 1.2%) that prolonged survival of C. albicans-infected nematodes and inhibited in vivo filamentation of C. albicans. Two compounds identified in the screen, caffeic acid phenethyl ester, a major active component of honeybee propolis, and the fluoroquinolone agent enoxacin exhibited antifungal activity in a murine model of candidiasis. The whole-animal C. elegans assay may help to study the molecular basis of C. albicans pathogenesis and identify antifungal compounds that most likely would not be identified by in vitro screens that target fungal growth. Compounds identified in the screen that affect the virulence of Candida in vivo can potentially be used as "probe compounds" and may have antifungal activity against other fungi.
Conflict of interest statement
Figures







References
-
- Ewbank JJ. Tackling both sides of the host-pathogen equation with Caenorhabditis elegans . Microbes Infect. 2002;4:247–256. - PubMed
-
- Slavin M, Fastenau J, Sukarom I, Mavros P, Crowley S, et al. Burden of hospitalization of patients with Candida and Aspergillus infections in Australia. Int J Infect Dis. 2004;8:111–120. - PubMed
-
- Rentz AM, Halpern MT, Bowden R. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin Infect Dis. 1998;27:781–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

