HER2 therapy. HER2 (ERBB2): functional diversity from structurally conserved building blocks
- PMID: 17274834
- PMCID: PMC1851388
- DOI: 10.1186/bcr1633
HER2 therapy. HER2 (ERBB2): functional diversity from structurally conserved building blocks
Abstract
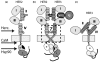
EGFR-type receptor tyrosine kinases achieve a broad spectrum of cellular responses by utilizing a set of structurally conserved building blocks. Based on available crystal structures and biochemical information, significant new insights have emerged into modes of receptor control, its deregulation in cancer, and the nuances that differentiate the four human receptors. This review gives an overview of current models of the control of receptor activity with a special emphasis on HER2 and HER3.
Figures
References
-
- Rubin I, Yarden Y. The basic biology of HER2. AnnOncol. 2001;(12 Suppl 1):S3–S8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

